Enterovirus 71 VP1 Protein Regulates Viral Replication in SH-SY5Y Cells via the mTOR Autophagy Signaling Pathway
- PMID: 31861844
- PMCID: PMC7019657
- DOI: 10.3390/v12010011
Enterovirus 71 VP1 Protein Regulates Viral Replication in SH-SY5Y Cells via the mTOR Autophagy Signaling Pathway
Abstract
Background: Enterovirus 71 (EV71) is the main pathogen that causes severe hand, foot, and mouth disease with fatal neurological complications. However, its neurovirulence mechanism is still unclear. Candidate virulence sites were screened out at structural protein VP1, but the function of these candidate virulence sites remains unclear. Several studies have shown that autophagy is associated with viral replication. However, the relationship between VP1 and autophagy in human neurons has not been studied.
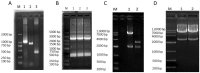
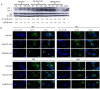
Methods: A recombinant virus-SDLY107-VP1, obtained by replacing the VP1 full-length gene of the SDLY107 strain with the VP1 full-length gene of the attenuated strain SDJN2015-01-was constructed and tested for replication and virulence. We then tested the effect of the recombinant virus on autophagy in nerve cells. The effect of autophagy on virus replication was detected by western blot and plaque test. Finally, the changes of mTOR signaling molecules during EV71 infection and the effect of mTOR on virus replication at the RNA level were detected.
Results: Viral recombination triggered virulence attenuation. The replication ability of recombinant virus SDLY107-VP1 was significantly weaker than that of the parent strain SDLY107. The SDLY107 strain could inhibit autophagic flux and led to accumulation of autophagosomes, while the SDLY107-VP1 strain could not cause autophagosome accumulation. The synthesis of EV71 RNA was inhibited by inhibiting mTOR.
Conclusions: Replacement of VP1 weakened the replication ability of virulent strains and reduced the level of autophagy in nerve cells. This autophagy facilitates the replication of virulent strains in nerve cells. VP1 is an important neurovirulence determinant of EV71, which affects virus replication by regulating cell autophagy. mTOR is a key molecule in this type of autophagy.
Keywords: EV71; VP1; autophagy; virus replication.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures

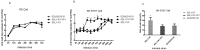


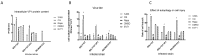
Similar articles
-
EV71 5'UTR interacts with 3D protein affecting replication through the AKT-mTOR pathway.Virol J. 2024 May 22;21(1):114. doi: 10.1186/s12985-024-02385-z. Virol J. 2024. PMID: 38778344 Free PMC article.
-
Nonstructural protein 2A modulates replication and virulence of enterovirus 71.Virus Res. 2018 Jan 15;244:262-269. doi: 10.1016/j.virusres.2017.11.023. Epub 2017 Nov 21. Virus Res. 2018. PMID: 29175108
-
Enterovirus 71-induced autophagy detected in vitro and in vivo promotes viral replication.J Med Virol. 2009 Jul;81(7):1241-52. doi: 10.1002/jmv.21502. J Med Virol. 2009. PMID: 19475621 Free PMC article.
-
Update on enterovirus 71 infection.Curr Opin Virol. 2014 Apr;5:98-104. doi: 10.1016/j.coviro.2014.03.007. Epub 2014 Apr 12. Curr Opin Virol. 2014. PMID: 24727707 Review.
-
[Molecular Mechanism of Action of hnRNP K and RTN3 in the Replication of Enterovirus 71].Bing Du Xue Bao. 2015 Mar;31(2):197-200. Bing Du Xue Bao. 2015. PMID: 26164948 Review. Chinese.
Cited by
-
Dysregulated autophagy contributes to the pathogenesis of enterovirus A71 infection.Cell Biosci. 2020 Dec 9;10(1):142. doi: 10.1186/s13578-020-00503-2. Cell Biosci. 2020. PMID: 33298183 Free PMC article. Review.
-
Candida albicans-enteric viral interactions-The prostaglandin E2 connection and host immune responses.iScience. 2022 Dec 24;26(1):105870. doi: 10.1016/j.isci.2022.105870. eCollection 2023 Jan 20. iScience. 2022. PMID: 36647379 Free PMC article. Review.
-
TLR4 signalling: the key to controlling EV71 replication and inflammatory response.Front Cell Infect Microbiol. 2024 Jun 13;14:1393680. doi: 10.3389/fcimb.2024.1393680. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38938877 Free PMC article.
-
Insight into the Life Cycle of Enterovirus-A71.Viruses. 2025 Jan 27;17(2):181. doi: 10.3390/v17020181. Viruses. 2025. PMID: 40006936 Free PMC article. Review.
-
Severe pneumonia and pathogenic damage in human airway epithelium caused by Coxsackievirus B4.Emerg Microbes Infect. 2023 Dec;12(2):2261560. doi: 10.1080/22221751.2023.2261560. Epub 2023 Sep 27. Emerg Microbes Infect. 2023. PMID: 37725516 Free PMC article.
References
-
- Chan L.G., Parashar U.D., Lye M.S., Ong F.G., Zaki S.R., Alexander J.P., Ho K.K., Han L.L., Pallansch M.A., Suleiman A.B., et al. Deaths of children during an outbreak of hand, foot, and mouth disease in sarawak, malaysia: Clinical and pathological characteristics of the disease. For the Outbreak Study Group. Clin. Infect. Dis. 2000;31:678–683. doi: 10.1086/314032. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

