Application of Graphene-Based Materials for Detection of Nitrate and Nitrite in Water-A Review
- PMID: 31861855
- PMCID: PMC6983230
- DOI: 10.3390/s20010054
Application of Graphene-Based Materials for Detection of Nitrate and Nitrite in Water-A Review
Abstract
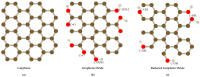
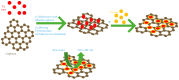
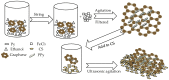
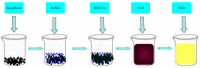
Nitrite and nitrate are widely found in various water environments but the potential toxicity of nitrite and nitrate poses a great threat to human health. Recently, many methods have been developed to detect nitrate and nitrite in water. One of them is to use graphene-based materials. Graphene is a two-dimensional carbon nano-material with sp2 hybrid orbital, which has a large surface area and excellent conductivity and electron transfer ability. It is widely used for modifying electrodes for electrochemical sensors. Graphene based electrochemical sensors have the advantages of being low cost, effective and efficient for nitrite and nitrate detection. This paper reviews the application of graphene-based nanomaterials for electrochemical detection of nitrate and nitrite in water. The properties and advantages of the electrodes were modified by graphene, graphene oxide and reduced graphene oxide nanocomposite in the development of nitrite sensors are discussed in detail. Based on the review, the paper summarizes the working conditions and performance of different sensors, including working potential, pH, detection range, detection limit, sensitivity, reproducibility, repeatability and long-term stability. Furthermore, the challenges and suggestions for future research on the application of graphene-based nanocomposite electrochemical sensors for nitrite detection are also highlighted.
Keywords: electrochemical sensing; graphene; graphene oxide; nitrate; nitrite; reduced graphene oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
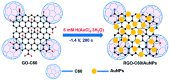
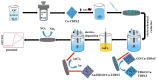
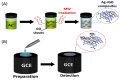

Similar articles
-
Composite of Cu metal nanoparticles-multiwall carbon nanotubes-reduced graphene oxide as a novel and high performance platform of the electrochemical sensor for simultaneous determination of nitrite and nitrate.J Hazard Mater. 2017 Feb 15;324(Pt B):762-772. doi: 10.1016/j.jhazmat.2016.11.055. Epub 2016 Nov 21. J Hazard Mater. 2017. PMID: 27894754
-
A simple manually rotated paper-based analytical device with electrochemical sensors for the determination of nitrite and nitrate.Talanta. 2025 Sep 1;292:127919. doi: 10.1016/j.talanta.2025.127919. Epub 2025 Mar 11. Talanta. 2025. PMID: 40107197
-
Strategies and Applications of Graphene and Its Derivatives-Based Electrochemical Sensors in Cancer Diagnosis.Molecules. 2023 Sep 20;28(18):6719. doi: 10.3390/molecules28186719. Molecules. 2023. PMID: 37764496 Free PMC article. Review.
-
Ag-CeO2 Based on Electrochemical Sensor for High-Efficient On-Site Detection of Nitrite in Aquaculture Water and Beverages.Molecules. 2024 Jun 4;29(11):2644. doi: 10.3390/molecules29112644. Molecules. 2024. PMID: 38893519 Free PMC article.
-
Graphene-family materials in electrochemical aptasensors.Anal Bioanal Chem. 2021 Jan;413(3):673-699. doi: 10.1007/s00216-020-02915-y. Epub 2020 Sep 17. Anal Bioanal Chem. 2021. PMID: 32939567 Review.
Cited by
-
Heterojunctions of rGO/Metal Oxide Nanocomposites as Promising Gas-Sensing Materials-A Review.Nanomaterials (Basel). 2022 Jul 1;12(13):2278. doi: 10.3390/nano12132278. Nanomaterials (Basel). 2022. PMID: 35808113 Free PMC article. Review.
-
The Role of Silver Nanoparticles in Electrochemical Sensors for Aquatic Environmental Analysis.Sensors (Basel). 2023 Apr 2;23(7):3692. doi: 10.3390/s23073692. Sensors (Basel). 2023. PMID: 37050752 Free PMC article. Review.
-
Emerging Two-Dimensional Materials-Based Electrochemical Sensors for Human Health and Environment Applications.Nanomaterials (Basel). 2023 Feb 20;13(4):780. doi: 10.3390/nano13040780. Nanomaterials (Basel). 2023. PMID: 36839148 Free PMC article. Review.
-
Sex-reversal and Histopathological Assessment of Potential Endocrine-Disrupting Effects of Graphene Oxide on Japanese medaka (Oryzias latipes) Larvae.Chemosphere. 2021 Sep;279:130768. doi: 10.1016/j.chemosphere.2021.130768. Epub 2021 May 17. Chemosphere. 2021. PMID: 34134430 Free PMC article.
-
Optimizing Carbon Structures in Laser-Induced Graphene Electrodes Using Design of Experiments for Enhanced Electrochemical Sensing Characteristics.ACS Appl Mater Interfaces. 2024 Nov 27;16(47):65489-65502. doi: 10.1021/acsami.4c13124. Epub 2024 Nov 14. ACS Appl Mater Interfaces. 2024. PMID: 39539231 Free PMC article.
References
-
- Yu J., Wang Z., Wang X., Xu J., Jia J. Study on Mechanism Experiments and Evaluation Methods for Water Eutrophication. J. Chem. 2017;2017:7. doi: 10.1155/2017/2036035. - DOI
-
- Fu H., Tu S., Huang G., Xie Q., Chen T., Gao P. Research on distribution characteristics of inorganic nitrogen in Yundang Lake: For prevention and control of the red tide. J. Nat. Dis. 2015;24:243–249.
-
- Lucchetti R., Onotri L., Clarizia L., Di Natale F., Di Somma I., Andreozzi R., Marotta R. Removal of nitrate and simultaneous hydrogen generation through photocatalytic reforming of glycerol over “in situ” prepared zero-valent nano copper/P25. Appl. Catal. B Environ. 2017;202:539–549. doi: 10.1016/j.apcatb.2016.09.043. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

