Impact Effect of Methyl Tertiary-Butyl Ether "Twelve Months Vapor Inhalation Study in Rats"
- PMID: 31861902
- PMCID: PMC7168921
- DOI: 10.3390/biology9010002
Impact Effect of Methyl Tertiary-Butyl Ether "Twelve Months Vapor Inhalation Study in Rats"
Abstract
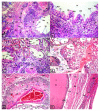
We investigated the early risk of developing cancer by inhalation of low doses (60 µL/day) of methyl tertiary butyl ether (MTBE) vapors using protein SDS-PAGE and LC-MS/MS analysis of rat sera. Furthermore, histological alterations were assessed in the trachea and lungs of 60 adult male Wistar rats. SDS-PAGE of blood sera showed three protein bands corresponding to 29, 28, and 21 kDa. Mass spectroscopy was used to identify these three bands. The upper and middle protein bands showed homology to carbonic anhydrase 2 (CA II), whereas the lower protein band showed homology with peroxiredoxin 2. We found that exposure to MTBE resulted in histopathological alterations in the trachea and the lungs. The histological anomalies of trachea and lung showed that the lumen of trachea, bronchi, and air alveoli packed with free and necrotic epithelial cells (epithelialization). The tracheal lamina propria of lung demonstrated aggregation of lymphoid cells, lymphoid hyperplasia, hemorrhage, adenomas, fibroid degeneration, steatosis, foam cells, severe inflammatory cells with monocytic infiltration, edema, hemorrhage. Occluded, congested, and hypertrophied lung arteries in addition, degenerated thyroid follicles, were observed. The hyaline cartilage displayed degeneration, deformation, and abnormal protrusion. In conclusion, our results suggest that inhalation of very low concentrations of the gasoline additive MTBE could induce an increase in protein levels and resulted in histopathological alterations of the trachea and the lungs.
Keywords: LC-MS/MS analysis; MTBE; cancer biomarker; histopathology.
Conflict of interest statement
The authors declare no conflict of interest in the assessment of results from this experiment.
Figures




References
-
- Komex, International Ltd. Water Quality Guidelines for Methyl Tertiary Butyl Ether (MTBE) British Colombia Ministry of Environment, Lands & Parks Water Management Branch; Alberta, BC, Canada: 2001.
-
- Fowler D.R. Master’s Thesis. The Evergreen State College; Olympia, WA, USA: Mar, 2011. An Analysis and Comparison of Methyl Tertiary Butyl Ether’s (MTBE’S) Use as a Fuel Oxygenate against the Tenets of the Precautionary Principle.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

