Activity and Affinity of Pin1 Variants
- PMID: 31861908
- PMCID: PMC6983177
- DOI: 10.3390/molecules25010036
Activity and Affinity of Pin1 Variants
Abstract
Pin1 is a peptidyl-prolyl isomerase responsible for isomerizing phosphorylated S/T-P motifs. Pin1 has two domains that each have a distinct ligand binding site, but only its PPIase domain has catalytic activity. Vast evidence supports interdomain allostery of Pin1, with binding of a ligand to its regulatory WW domain impacting activity in the PPIase domain. Many diverse studies have made mutations in Pin1 in order to elucidate interactions that are responsible for ligand binding, isomerase activity, and interdomain allostery. Here, we summarize these mutations and their impact on Pin1's structure and function.
Keywords: PPIase domain; WW domain; activity; affinity; mutants; pin1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

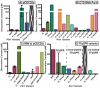
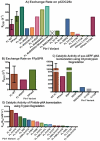

References
-
- Yaffe M.B., Schutkowski M., Shen M., Zhou X.Z., Stukenberg P.T., Rahfeld J.U., Xu J., Kuang J., Kirschner M.W., Fischer G., et al. Sequence-specific and phosphorylation dependent proline isomerization: A potential mitotic regulatory mechanism. Science. 1997;278:1957–1960. doi: 10.1126/science.278.5345.1957. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

