Upregulation of PUM1 Expression in Preeclampsia Impairs Trophoblast Invasion by Negatively Regulating the Expression of the lncRNA HOTAIR
- PMID: 31862314
- PMCID: PMC7001091
- DOI: 10.1016/j.ymthe.2019.11.025
Upregulation of PUM1 Expression in Preeclampsia Impairs Trophoblast Invasion by Negatively Regulating the Expression of the lncRNA HOTAIR
Abstract
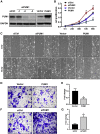
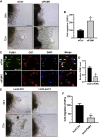
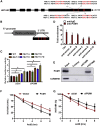
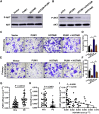
Pumilio (PUM) proteins are members of a highly conserved RNA-binding protein family that posttranscriptionally regulate gene expression in many organisms. However, their roles in the placenta are unclear. In the present study, we report the requirement for the PUM homolog 1 (PUM1) gene in preeclampsia (PE). Immunofluorescence and immunohistochemical data showed that PUM1 was highly expressed in human placental villi from women with PE compared to healthy controls (HCs). Further, PUM1 overexpression repressed, and knockdown enhanced, the invasion and proliferation of trophoblasts. Interestingly, PUM1 knockdown promoted trophoblast invasion in a villous explant culture model, while PUM1 overexpression repressed these effects. Furthermore, lncRNA transcriptome sequencing coupled with RNA immunoprecipitation (RIP) revealed that PUM1 inhibits trophoblast invasion in PE by downregulating the expression of lncRNA HOTAIR. Moreover, PUM1 regulates HOTAIR expression via a posttranscriptional mechanism. Using RNA-protein pull-down and mRNA stability assays, we identified PUM1 as a specific binding partner that decreased the half-life of HOTAIR and lowered the steady-state level of HOTAIR expression, suggesting a novel posttranscriptional regulatory mechanism. Collectively, these findings identified a novel RNA regulatory mechanism, revealing a new pathway governing the regulation of PUM1/HOTAIR in trophoblast invasion in the pathogenesis of PE.
Keywords: HOTAIR; PUM1; lncRNA stability; preeclampsia; trophoblast invasion.
Copyright © 2019 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Elevated Tristetraprolin Impairs Trophoblast Invasion in Women with Recurrent Miscarriage by Destabilization of HOTAIR.Mol Ther Nucleic Acids. 2018 Sep 7;12:600-609. doi: 10.1016/j.omtn.2018.07.001. Epub 2018 Jul 6. Mol Ther Nucleic Acids. 2018. Retraction in: Mol Ther Nucleic Acids. 2022 May 10;28:599. doi: 10.1016/j.omtn.2022.05.004. PMID: 30195796 Free PMC article. Retracted.
-
Long non-coding RNA HOTAIR modulates the progression of preeclampsia through inhibiting miR-106 in an EZH2-dependent manner.Life Sci. 2020 Jul 15;253:117668. doi: 10.1016/j.lfs.2020.117668. Epub 2020 Apr 19. Life Sci. 2020. PMID: 32320706
-
Upregulated long noncoding RNA Linc00261 in pre-eclampsia and its effect on trophoblast invasion and migration via regulating miR-558/TIMP4 signaling pathway.J Cell Biochem. 2019 Aug;120(8):13243-13253. doi: 10.1002/jcb.28598. Epub 2019 Mar 19. J Cell Biochem. 2019. PMID: 30891826
-
Overexpression of lncRNA TCL6 promotes preeclampsia progression by regulating PTEN.Eur Rev Med Pharmacol Sci. 2019 May;23(10):4066-4072. doi: 10.26355/eurrev_201905_17907. Eur Rev Med Pharmacol Sci. 2019. PMID: 31173275 Review.
-
Dysregulation of LncRNAs in Placenta and Pathogenesis of Preeclampsia.Curr Drug Targets. 2017;18(10):1165-1170. doi: 10.2174/1389450118666170404160000. Curr Drug Targets. 2017. PMID: 28382860 Review.
Cited by
-
Silencing of LncRNA SNHG6 protects trophoblast cells through regulating miR-101-3p/OTUD3 axis in unexplained recurrent spontaneous abortion.J Mol Histol. 2022 Dec;53(6):871-882. doi: 10.1007/s10735-022-10102-9. Epub 2022 Sep 29. J Mol Histol. 2022. PMID: 36173586
-
Depleting long noncoding RNA HOTAIR attenuates chronic myelocytic leukemia progression by binding to DNA methyltransferase 1 and inhibiting PTEN gene promoter methylation.Cell Death Dis. 2021 May 3;12(5):440. doi: 10.1038/s41419-021-03637-4. Cell Death Dis. 2021. PMID: 33941772 Free PMC article.
-
Roles of noncoding RNAs in preeclampsia.Reprod Biol Endocrinol. 2021 Jul 2;19(1):100. doi: 10.1186/s12958-021-00783-4. Reprod Biol Endocrinol. 2021. PMID: 34215266 Free PMC article. Review.
-
The long noncoding RNA TARID regulates the CXCL3/ERK/MAPK pathway in trophoblasts and is associated with preeclampsia.Reprod Biol Endocrinol. 2022 Nov 19;20(1):159. doi: 10.1186/s12958-022-01036-8. Reprod Biol Endocrinol. 2022. PMID: 36401313 Free PMC article.
-
Pristimerin Suppresses Trophoblast Cell Epithelial-Mesenchymal Transition via miR-542-5p/EGFR Axis.Drug Des Devel Ther. 2020 Nov 2;14:4659-4670. doi: 10.2147/DDDT.S274595. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33173276 Free PMC article.
References
-
- Sharma S., Godbole G., Modi D. Decidual Control of Trophoblast Invasion. Am. J. Reprod. Immunol. 2016;75:341–350. - PubMed
-
- Aplin J.D. Implantation, trophoblast differentiation and haemochorial placentation: mechanistic evidence in vivo and in vitro. J. Cell Sci. 1991;99:681–692. - PubMed
-
- Strickland S., Richards W.G. Invasion of the trophoblasts. Cell. 1992;71:355–357. - PubMed
-
- Lain K.Y., Roberts J.M. Contemporary concepts of the pathogenesis and management of preeclampsia. JAMA. 2002;287:3183–3186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

