Radiohybrid Ligands: A Novel Tracer Concept Exemplified by 18F- or 68Ga-Labeled rhPSMA Inhibitors
- PMID: 31862804
- PMCID: PMC7198388
- DOI: 10.2967/jnumed.119.234922
Radiohybrid Ligands: A Novel Tracer Concept Exemplified by 18F- or 68Ga-Labeled rhPSMA Inhibitors
Abstract
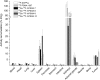
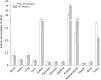
When we critically assess the reason for the current dominance of 68Ga-labeled peptides and peptide-like ligands in radiopharmacy and nuclear medicine, we have to conclude that the major advantage of such radiopharmaceuticals is the apparent lack of suitable 18F-labeling technologies with proven clinical relevance. To prepare and to subsequently perform a clinical proof-of-concept study on the general suitability of silicon-fluoride-acceptor (SiFA)-conjugated radiopharmaceuticals, we developed inhibitors of the prostate-specific membrane antigen (PSMA) that are labeled by isotopic exchange (IE). To compensate for the pronounced lipophilicity of the SiFA unit, we used metal chelates, conjugated in close proximity to SiFA. Six different radiohybrid PSMA ligands (rhPSMA ligands) were evaluated and compared with the commonly used 18F-PSMA inhibitors 18F-DCFPyL and 18F-PSMA-1007. Methods: All inhibitors were synthesized by solid-phase peptide synthesis. Human serum albumin binding was measured by affinity high-performance liquid chromatography, whereas the lipophilicity of each tracer was determined by the n-octanol/buffer method. In vitro studies (IC50, internalization) were performed on LNCaP cells. Biodistribution studies were conducted on LNCaP tumor-bearing male CB-17 SCID mice. Results: On the laboratory scale (starting activities, 0.2-9.0 GBq), labeling of 18F-rhPSMA-5 to -10 by IE was completed in < 20 min (radiochemical yields, 58% ± 9%; radiochemical purity, >97%) with molar activities of 12-60 GBq/μmol. All rhPSMAs showed low nanomolar affinity and high internalization by PSMA-expressing cells when compared with the reference radiopharmaceuticals, medium-to-low lipophilicity, and high human serum albumin binding. Biodistribution studies in LNCaP tumor-bearing mice revealed high tumor uptake, sufficiently fast clearance kinetics from blood, low hepatobiliary excretion, fast renal excretion, and very low uptake of 18F activity in bone. Conclusion: The novel 18F-rhPSMA radiopharmaceuticals developed under the radiohybrid concept show equal or better targeting characteristics than the established 18F-PSMA tracers 18F-DCFPyL and 18F-PSMA-1007. The unparalleled simplicity of production, the possibility to produce the identical 68Ga-labeled 19F-68Ga-rhPSMA tracers, and the possibility to extend this concept to true theranostic radiohybrid radiopharmaceuticals, such as F-Lu-rhPSMA, are unique features of these radiopharmaceuticals.
Keywords: 18F; PSMA; prostate cancer; radiohybrid.
© 2020 by the Society of Nuclear Medicine and Molecular Imaging.
Figures



References
-
- Schwaiger M, Wester HJ. How many PET tracers do we need? J Nucl Med. 2011;52(suppl 2):36S–41S. - PubMed
-
- Maecke HR, André JP. 68Ga-PET radiopharmacy: A generator-based alternative to 18F-radiopharmacy. Ernst Schering Res Found Workshop. 2007;62:215–242. - PubMed
-
- Dornan MH, Simard JM, Leblond A, et al. Simplified and robust one-step radiosynthesis of [18F]DCFPyL via direct radiofluorination and cartridge-based purification. J Labelled Comp Radiopharm. 2018;61:757–763. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
