Categorical Biases in Human Occipitoparietal Cortex
- PMID: 31862856
- PMCID: PMC6975303
- DOI: 10.1523/JNEUROSCI.2700-19.2019
Categorical Biases in Human Occipitoparietal Cortex
Abstract
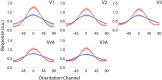
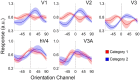
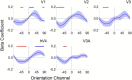
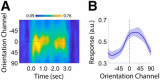
Categorization allows organisms to generalize existing knowledge to novel stimuli and to discriminate between physically similar yet conceptually different stimuli. Humans, nonhuman primates, and rodents can readily learn arbitrary categories defined by low-level visual features, and learning distorts perceptual sensitivity for category-defining features such that differences between physically similar yet categorically distinct exemplars are enhanced, whereas differences between equally similar but categorically identical stimuli are reduced. We report a possible basis for these distortions in human occipitoparietal cortex. In three experiments, we used an inverted encoding model to recover population-level representations of stimuli from multivoxel and multielectrode patterns of human brain activity while human participants (both sexes) classified continuous stimulus sets into discrete groups. In each experiment, reconstructed representations of to-be-categorized stimuli were systematically biased toward the center of the appropriate category. These biases were largest for exemplars near a category boundary, predicted participants' overt category judgments, emerged shortly after stimulus onset, and could not be explained by mechanisms of response selection or motor preparation. Collectively, our findings suggest that category learning can influence processing at the earliest stages of cortical visual processing.SIGNIFICANCE STATEMENT Category learning enhances perceptual sensitivity for physically similar yet categorically different stimuli. We report a possible mechanism for these changes in human occipitoparietal cortex. In three experiments, we used an inverted encoding model to recover population-level representations of stimuli from multivariate patterns in occipitoparietal cortex while participants categorized sets of continuous stimuli into discrete groups. The recovered representations were systematically biased by category membership, with larger biases for exemplars adjacent to a category boundary. These results suggest that mechanisms of categorization shape information processing at the earliest stages of the visual system.
Keywords: EEG; categorization; fMRI; human; occipital cortex.
Copyright © 2020 the authors.
Figures













Comment in
-
Complementary Brain Signals for Categorical Decisions.J Neurosci. 2020 Jul 22;40(30):5706-5708. doi: 10.1523/JNEUROSCI.0785-20.2020. J Neurosci. 2020. PMID: 32699153 Free PMC article. No abstract available.
Similar articles
-
Parietal-Occipital Interactions Underlying Control- and Representation-Related Processes in Working Memory for Nonspatial Visual Features.J Neurosci. 2018 May 2;38(18):4357-4366. doi: 10.1523/JNEUROSCI.2747-17.2018. Epub 2018 Apr 10. J Neurosci. 2018. PMID: 29636395 Free PMC article.
-
Roles of Category, Shape, and Spatial Frequency in Shaping Animal and Tool Selectivity in the Occipitotemporal Cortex.J Neurosci. 2020 Jul 15;40(29):5644-5657. doi: 10.1523/JNEUROSCI.3064-19.2020. Epub 2020 Jun 11. J Neurosci. 2020. PMID: 32527983 Free PMC article.
-
Category Learning Selectively Enhances Representations of Boundary-Adjacent Exemplars in Early Visual Cortex.J Neurosci. 2024 Jan 17;44(3):e1039232023. doi: 10.1523/JNEUROSCI.1039-23.2023. J Neurosci. 2024. PMID: 37968121 Free PMC article.
-
Neuronal Mechanisms of Visual Categorization: An Abstract View on Decision Making.Annu Rev Neurosci. 2016 Jul 8;39:129-47. doi: 10.1146/annurev-neuro-071714-033919. Epub 2016 Apr 8. Annu Rev Neurosci. 2016. PMID: 27070552 Review.
-
Prototypes, exemplars, and the natural history of categorization.Psychon Bull Rev. 2014 Apr;21(2):312-31. doi: 10.3758/s13423-013-0506-0. Psychon Bull Rev. 2014. PMID: 24005828 Free PMC article. Review.
Cited by
-
Visual and Semantic Representations Predict Subsequent Memory in Perceptual and Conceptual Memory Tests.Cereb Cortex. 2021 Jan 5;31(2):974-992. doi: 10.1093/cercor/bhaa269. Cereb Cortex. 2021. PMID: 32935833 Free PMC article.
-
Is Categorization in Visual Working Memory a Way to Reduce Mental Effort? A Pupillometry Study.Cogn Sci. 2022 Sep;46(9):e13194. doi: 10.1111/cogs.13194. Cogn Sci. 2022. PMID: 36070854 Free PMC article.
-
Interactions Between Visual Working Memory, Attention, and Color Categories: A Pupillometry Study.J Cogn. 2022 Feb 7;5(1):16. doi: 10.5334/joc.208. eCollection 2022. J Cogn. 2022. PMID: 36072094 Free PMC article.
-
Improving the validity of neuroimaging decoding tests of invariant and configural neural representation.PLoS Comput Biol. 2023 Jan 23;19(1):e1010819. doi: 10.1371/journal.pcbi.1010819. eCollection 2023 Jan. PLoS Comput Biol. 2023. PMID: 36689555 Free PMC article.
-
A direct neural signature of serial dependence in working memory.Elife. 2025 Jun 23;13:RP99478. doi: 10.7554/eLife.99478. Elife. 2025. PMID: 40549432 Free PMC article.
References
-
- Blankertz B, Lemm S, Treder M, Haufe S, Muller KR (2011) Single-trial analysis and classification of ERP components - a tutorial. Neuroimage 56:814–825. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
