Paclitaxel induces lymphatic endothelial cells autophagy to promote metastasis
- PMID: 31863036
- PMCID: PMC6925245
- DOI: 10.1038/s41419-019-2181-1
Paclitaxel induces lymphatic endothelial cells autophagy to promote metastasis
Abstract
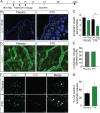
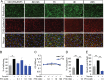
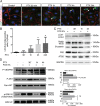
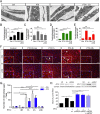
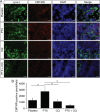
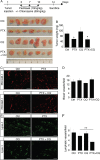
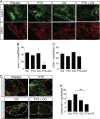
Cytotoxic therapy for breast cancer inhibits the growth of primary tumors, but promotes metastasis to the sentinel lymph nodes through the lymphatic system. However, the effect of first-line chemotherapy on the lymphatic endothelium has been poorly investigated. In this study, we determined that paclitaxel, the anti-cancer drug approved for the treatment of metastatic or locally advanced breast cancer, induces lymphatic endothelial cell (LEC) autophagy to increase metastases. While paclitaxel treatment was largely efficacious in inhibiting LEC adhesion, it had no effect on cell survival. Paclitaxel inhibited LEC migration and branch point formation by inducing an autophagy mechanism independent of Akt phosphorylation. In vivo, paclitaxel mediated a higher permeability of lymphatic endothelium to tumor cells and this effect was reversed by chloroquine, an autophagy-lysosome inhibitor. Despite a strong effect on reducing tumor size, paclitaxel significantly increased metastasis to the sentinel lymph nodes. This effect was restricted to a lymphatic dissemination, as chemotherapy did not affect the blood endothelium. Taken together, our findings suggest that the lymphatic system resists to chemotherapy through an autophagy mechanism to promote malignant progression and metastatic lesions. This study paves the way for new combinative therapies aimed at reducing the number of metastases.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

