Allergen-induced anxiety-like behavior is associated with disruption of medial prefrontal cortex - amygdala circuit
- PMID: 31863052
- PMCID: PMC6925103
- DOI: 10.1038/s41598-019-55539-3
Allergen-induced anxiety-like behavior is associated with disruption of medial prefrontal cortex - amygdala circuit
Abstract
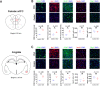
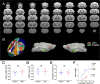
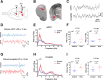
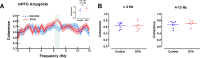
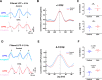
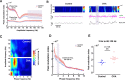
Anxiety is prevalent in asthma, and is associated with disease severity and poor quality of life. However, no study to date provides direct experimental evidence for the effect of allergic inflammation on the structure and function of medial prefrontal cortex (mPFC) and amygdala, which are essential regions for modulating anxiety and its behavioral expression. We assessed the impact of ovalbumin (OVA)-induced allergic inflammation on the appearance of anxiety-like behavior, mPFC and amygdala volumes using MRI, and the mPFC-amygdala circuit activity in sensitized rats. Our findings exhibited that the OVA challenge in sensitized rats induced anxiety-like behavior, and led to more activated microglia and astrocytes in the mPFC and amygdala. We also found a negative correlation between anxiety-like behavior and amygdala volume. Moreover, OVA challenge in sensitized rats was associated with increases in mPFC and amygdala activity, elevation of amygdala delta-gamma coupling, and the enhancement of functional connectivity within mPFC-amygdala circuit - accompanied by an inverted direction of information transferred from the amygdala to the mPFC. We indicated that disrupting the dynamic interactions of the mPFC-amygdala circuit may contribute to the induction of anxiety-related behaviors with asthma. These findings could provide new insight to clarify the underlying mechanisms of allergic inflammation-induced psychiatric disorders related to asthma.
Conflict of interest statement
The authors declare no competing interests.
Figures

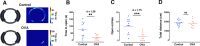
Similar articles
-
Early-life stress induces anxiety-like behaviors and activity imbalances in the medial prefrontal cortex and amygdala in adult rats.Eur J Neurosci. 2015 Feb;41(4):442-53. doi: 10.1111/ejn.12825. Epub 2015 Jan 10. Eur J Neurosci. 2015. PMID: 25581710
-
Allergen Induces Depression-like Behavior in Association with Altered Prefrontal-hippocampal Circuit in Male Rats.Neuroscience. 2023 Aug 1;524:21-36. doi: 10.1016/j.neuroscience.2023.05.034. Epub 2023 Jun 5. Neuroscience. 2023. PMID: 37286161
-
Corticosteroid treatment attenuates anxiety and mPFC-amygdala circuit dysfunction in allergic asthma.Life Sci. 2023 Feb 15;315:121373. doi: 10.1016/j.lfs.2023.121373. Epub 2023 Jan 5. Life Sci. 2023. PMID: 36621536
-
Amygdala hyper-connectivity in a mouse model of unpredictable early life stress.Transl Psychiatry. 2018 Feb 21;8(1):49. doi: 10.1038/s41398-018-0092-z. Transl Psychiatry. 2018. PMID: 29463821 Free PMC article. Review.
-
Differential Rearrangement of Excitatory Inputs to the Medial Prefrontal Cortex in Chronic Pain Models.Front Neural Circuits. 2021 Dec 24;15:791043. doi: 10.3389/fncir.2021.791043. eCollection 2021. Front Neural Circuits. 2021. PMID: 35002635 Free PMC article. Review.
Cited by
-
New Insights into the Pivotal Role of the Amygdala in Inflammation-Related Depression and Anxiety Disorder.Int J Mol Sci. 2022 Sep 21;23(19):11076. doi: 10.3390/ijms231911076. Int J Mol Sci. 2022. PMID: 36232376 Free PMC article. Review.
-
Amygdalar involvement in respiratory dysfunction.Front Physiol. 2024 Aug 28;15:1424889. doi: 10.3389/fphys.2024.1424889. eCollection 2024. Front Physiol. 2024. PMID: 39263625 Free PMC article. Review.
-
Liposaccharide-induced sustained mild inflammation fragments social behavior and alters basolateral amygdala activity.Psychopharmacology (Berl). 2023 Mar;240(3):647-671. doi: 10.1007/s00213-023-06308-8. Epub 2023 Jan 16. Psychopharmacology (Berl). 2023. PMID: 36645464
-
Fueling the fire in the lung-brain axis: The salience network connects allergen-provoked TH17 responses to psychological stress in asthma.Brain Behav Immun. 2025 Aug;128:276-288. doi: 10.1016/j.bbi.2025.04.004. Epub 2025 Apr 8. Brain Behav Immun. 2025. PMID: 40209864
-
Investigating Olfactory Sensory Neurons Facilitation For Aerobic Exercise-induced Spatial Memory Improvement.Basic Clin Neurosci. 2024 May-Jun;15(3):355-366. doi: 10.32598/bcn.2022.4029.1. Epub 2024 May 1. Basic Clin Neurosci. 2024. PMID: 39403352 Free PMC article.

