A prospective compound screening contest identified broader inhibitors for Sirtuin 1
- PMID: 31863054
- PMCID: PMC6925144
- DOI: 10.1038/s41598-019-55069-y
A prospective compound screening contest identified broader inhibitors for Sirtuin 1
Abstract
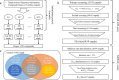
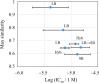
Potential inhibitors of a target biomolecule, NAD-dependent deacetylase Sirtuin 1, were identified by a contest-based approach, in which participants were asked to propose a prioritized list of 400 compounds from a designated compound library containing 2.5 million compounds using in silico methods and scoring. Our aim was to identify target enzyme inhibitors and to benchmark computer-aided drug discovery methods under the same experimental conditions. Collecting compound lists derived from various methods is advantageous for aggregating compounds with structurally diversified properties compared with the use of a single method. The inhibitory action on Sirtuin 1 of approximately half of the proposed compounds was experimentally accessed. Ultimately, seven structurally diverse compounds were identified.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Bender A, et al. Which aspects of HTS are empirically correlated with downstream success? Curr Opin Drug Discov Devel. 2008;11:327–337. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

