Controlling properties of human neural progenitor cells using 2D and 3D conductive polymer scaffolds
- PMID: 31863072
- PMCID: PMC6925212
- DOI: 10.1038/s41598-019-56021-w
Controlling properties of human neural progenitor cells using 2D and 3D conductive polymer scaffolds
Abstract
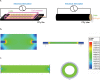
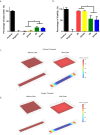
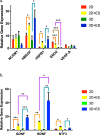
Human induced pluripotent stem cell-derived neural progenitor cells (hNPCs) are a promising cell source for stem cell transplantation to treat neurological diseases such as stroke and peripheral nerve injuries. However, there have been limited studies investigating how the dimensionality of the physical and electrical microenvironment affects hNPC function. In this study, we report the fabrication of two- and three-dimensional (2D and 3D respectively) constructs composed of a conductive polymer to compare the effect of electrical stimulation of hydrogel-immobilized hNPCs. The physical dimension (2D vs 3D) of stimulating platforms alone changed the hNPCs gene expression related to cell proliferation and metabolic pathways. The addition of electrical stimulation was critical in upregulating gene expression of neurotrophic factors that are important in regulating cell survival, synaptic remodeling, and nerve regeneration. This study demonstrates that the applied electrical field controls hNPC properties depending on the physical nature of stimulating platforms and cellular metabolic states. The ability to control hNPC functions can be beneficial in understanding mechanistic changes related to electrical modulation and devising novel treatment methods for neurological diseases.
Conflict of interest statement
There are no competing or conflicts of interest related to the work presented in this manuscript for the authors.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

