Identification of the 3-amino-3-carboxypropyl (acp) transferase enzyme responsible for acp3U formation at position 47 in Escherichia coli tRNAs
- PMID: 31863583
- PMCID: PMC7026641
- DOI: 10.1093/nar/gkz1191
Identification of the 3-amino-3-carboxypropyl (acp) transferase enzyme responsible for acp3U formation at position 47 in Escherichia coli tRNAs
Abstract
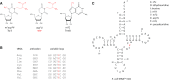
tRNAs from all domains of life contain modified nucleotides. However, even for the experimentally most thoroughly characterized model organism Escherichia coli not all tRNA modification enzymes are known. In particular, no enzyme has been found yet for introducing the acp3U modification at position 47 in the variable loop of eight E. coli tRNAs. Here we identify the so far functionally uncharacterized YfiP protein as the SAM-dependent 3-amino-3-carboxypropyl transferase catalyzing this modification and thereby extend the list of known tRNA modification enzymes in E. coli. Similar to the Tsr3 enzymes that introduce acp modifications at U or m1Ψ nucleotides in rRNAs this protein contains a DTW domain suggesting that acp transfer reactions to RNA nucleotides are a general function of DTW domain containing proteins. The introduction of the acp3U-47 modification in E. coli tRNAs is promoted by the presence of the m7G-46 modification as well as by growth in rich medium. However, a deletion of the enzymes responsible for the modifications at position 46 and 47 in the variable loop of E. coli tRNAs did not lead to a clearly discernible phenotype suggesting that these two modifications play only a minor role in ensuring the proper function of tRNAs in E. coli.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


Similar articles
-
acp³U: A Conserved RNA Modification with Lessons Yet to Unfold.Mol Cell Biol. 2025;45(6):238-245. doi: 10.1080/10985549.2024.2443138. Epub 2025 Jan 6. Mol Cell Biol. 2025. PMID: 39757918 Review.
-
Biogenesis and functions of aminocarboxypropyluridine in tRNA.Nat Commun. 2019 Dec 5;10(1):5542. doi: 10.1038/s41467-019-13525-3. Nat Commun. 2019. PMID: 31804502 Free PMC article.
-
Comparison of dissimilarity patterns of E coli, yeast and mammalian tRNAs.Biochimie. 1992 Apr;74(4):337-51. doi: 10.1016/0300-9084(92)90111-q. Biochimie. 1992. PMID: 1379077
-
The modification landscape of P. aeruginosa tRNAs.bioRxiv [Preprint]. 2024 Feb 21:2024.02.21.581370. doi: 10.1101/2024.02.21.581370. bioRxiv. 2024. Update in: RNA. 2024 Jul 16;30(8):1025-1040. doi: 10.1261/rna.080004.124. PMID: 38529508 Free PMC article. Updated. Preprint.
-
To be or not to be modified: Miscellaneous aspects influencing nucleotide modifications in tRNAs.IUBMB Life. 2019 Aug;71(8):1126-1140. doi: 10.1002/iub.2041. Epub 2019 Apr 1. IUBMB Life. 2019. PMID: 30932315 Free PMC article. Review.
Cited by
-
tRNA elbow modifications affect the tRNA pseudouridine synthase TruB and the methyltransferase TrmA.RNA. 2020 Sep;26(9):1131-1142. doi: 10.1261/rna.075473.120. Epub 2020 May 8. RNA. 2020. PMID: 32385137 Free PMC article.
-
Biochemical Pathways Leading to the Formation of Wyosine Derivatives in tRNA of Archaea.Biomolecules. 2020 Dec 2;10(12):1627. doi: 10.3390/biom10121627. Biomolecules. 2020. PMID: 33276555 Free PMC article. Review.
-
S-Adenosylmethionine: more than just a methyl donor.Nat Prod Rep. 2023 Sep 20;40(9):1521-1549. doi: 10.1039/d2np00086e. Nat Prod Rep. 2023. PMID: 36891755 Free PMC article. Review.
-
Naturally occurring modified ribonucleosides.Wiley Interdiscip Rev RNA. 2020 Sep;11(5):e1595. doi: 10.1002/wrna.1595. Epub 2020 Apr 16. Wiley Interdiscip Rev RNA. 2020. PMID: 32301288 Free PMC article. Review.
-
Beyond the Anticodon: tRNA Core Modifications and Their Impact on Structure, Translation and Stress Adaptation.Genes (Basel). 2024 Mar 19;15(3):374. doi: 10.3390/genes15030374. Genes (Basel). 2024. PMID: 38540433 Free PMC article. Review.
References
-
- Kowalak J.A., Bruenger E., Crain P.F., McCloskey J.A.. Identities and phylogenetic comparisons of posttranscriptional modifications in 16 S ribosomal RNA from Haloferax volcanii. J. Biol. Chem. 2000; 275:24484–24489. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

