Adapted formaldehyde gradient cross-linking protocol implicates human eIF3d and eIF3c, k and l subunits in the 43S and 48S pre-initiation complex assembly, respectively
- PMID: 31863585
- PMCID: PMC7039009
- DOI: 10.1093/nar/gkz1185
Adapted formaldehyde gradient cross-linking protocol implicates human eIF3d and eIF3c, k and l subunits in the 43S and 48S pre-initiation complex assembly, respectively
Abstract
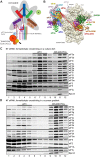
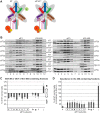
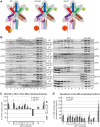
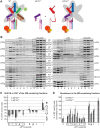
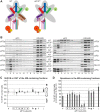
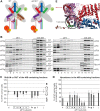
One of the key roles of the 12-subunit eukaryotic translation initiation factor 3 (eIF3) is to promote the formation of the 43S and 48S pre-initiation complexes (PICs). However, particular contributions of its individual subunits to these two critical initiation reactions remained obscure. Here, we adapted formaldehyde gradient cross-linking protocol to translation studies and investigated the efficiency of the 43S and 48S PIC assembly in knockdowns of individual subunits of human eIF3 known to produce various partial subcomplexes. We revealed that eIF3d constitutes an important intermolecular bridge between eIF3 and the 40S subunit as its elimination from the eIF3 holocomplex severely compromised the 43S PIC assembly. Similarly, subunits eIF3a, c and e were found to represent an important binding force driving eIF3 binding to the 40S subunit. In addition, we demonstrated that eIF3c, and eIF3k and l subunits alter the efficiency of mRNA recruitment to 43S PICs in an opposite manner. Whereas the eIF3c knockdown reduces it, downregulation of eIF3k or eIF3l increases mRNA recruitment, suggesting that the latter subunits possess a regulatory potential. Altogether this study provides new insights into the role of human eIF3 in the initial assembly steps of the translational machinery.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

