Thioredoxin targets are regulated in heterocysts of cyanobacterium Anabaena sp. PCC 7120 in a light-independent manner
- PMID: 31863668
- PMCID: PMC7242069
- DOI: 10.1093/jxb/erz561
Thioredoxin targets are regulated in heterocysts of cyanobacterium Anabaena sp. PCC 7120 in a light-independent manner
Abstract
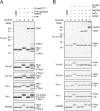
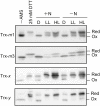
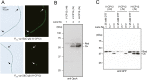
In the nitrogen-fixing cyanobacterium Anabaena sp. PCC 7120, glucose 6-phosphate dehydrogenase (G6PDH) plays an important role in producing the power for reducing nitrogenase under light conditions. Our previous study showed that thioredoxin suppresses G6PDH by reducing its activator protein OpcA, implying that G6PDH is inactivated under light conditions because thioredoxins are reduced by the photosynthetic electron transport system in cyanobacteria. To address how Anabaena sp. PCC 7120 maintains G6PDH activity even under light conditions when nitrogen fixation occurs, we investigated the redox regulation system in vegetative cells and specific nitrogen-fixing cells named heterocysts, individually. We found that thioredoxin target proteins were more oxidized in heterocysts than in vegetative cells under light conditions. Alterations in the redox regulation mechanism of heterocysts may affect the redox states of thioredoxin target proteins, including OpcA, so that G6PDH is activated in heterocysts even under light conditions.
Keywords: Anabaena; heterocyst; nitrogen fixation; oxidative pentose phosphate pathway; photosynthesis; redox regulation; thioredoxin.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Experimental Biology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures




Similar articles
-
Thioredoxin regulates G6PDH activity by changing redox states of OpcA in the nitrogen-fixing cyanobacterium Anabaena sp. PCC 7120.Biochem J. 2018 Mar 20;475(6):1091-1105. doi: 10.1042/BCJ20170869. Biochem J. 2018. PMID: 29440317
-
Involvement of thioredoxin on the scaffold activity of NifU in heterocyst cells of the diazotrophic cyanobacterium Anabaena sp. strain PCC 7120.J Biochem. 2015 Sep;158(3):253-61. doi: 10.1093/jb/mvv046. Epub 2015 May 6. J Biochem. 2015. PMID: 25953913
-
Thioredoxins and the redox modulation of glucose-6-phosphate dehydrogenase in Anabaena sp. strain PCC 7120 vegetative cells and heterocysts.J Bacteriol. 1984 Feb;157(2):681-3. doi: 10.1128/jb.157.2.681-683.1984. J Bacteriol. 1984. PMID: 6420395 Free PMC article.
-
Roles of DevBCA-like ABC transporters in the physiology of Anabaena sp. PCC 7120.Int J Med Microbiol. 2019 Jul;309(5):325-330. doi: 10.1016/j.ijmm.2019.04.005. Epub 2019 Apr 28. Int J Med Microbiol. 2019. PMID: 31133373 Review.
-
Genetic responses to carbon and nitrogen availability in Anabaena.Environ Microbiol. 2019 Jan;21(1):1-17. doi: 10.1111/1462-2920.14370. Epub 2018 Oct 16. Environ Microbiol. 2019. PMID: 30066380 Review.
Cited by
-
Depletion of m-type thioredoxin impairs photosynthesis, carbon fixation, and oxidative stress in cyanobacteria.Plant Physiol. 2021 Nov 3;187(3):1325-1340. doi: 10.1093/plphys/kiab321. Plant Physiol. 2021. PMID: 34618018 Free PMC article.
-
L-Lactate treatment by photosynthetic cyanobacteria expressing heterogeneous L-lactate dehydrogenase.Sci Rep. 2023 May 4;13(1):7249. doi: 10.1038/s41598-023-34289-3. Sci Rep. 2023. PMID: 37142758 Free PMC article.
-
Thioredoxin Dependent Changes in the Redox States of FurA from Anabaena sp. PCC 7120.Antioxidants (Basel). 2021 Jun 4;10(6):913. doi: 10.3390/antiox10060913. Antioxidants (Basel). 2021. PMID: 34199999 Free PMC article.
-
Thiol redox switches regulate the oligomeric state of cyanobacterial Rre1, RpaA and RpaB response regulators.FEBS Lett. 2022 Jun;596(12):1533-1543. doi: 10.1002/1873-3468.14340. Epub 2022 Apr 11. FEBS Lett. 2022. PMID: 35353903 Free PMC article.
-
Expanding the FurC (PerR) regulon in Anabaena (Nostoc) sp. PCC 7120: Genome-wide identification of novel direct targets uncovers FurC participation in central carbon metabolism regulation.PLoS One. 2023 Aug 7;18(8):e0289761. doi: 10.1371/journal.pone.0289761. eCollection 2023. PLoS One. 2023. PMID: 37549165 Free PMC article.
References
-
- Buchanan BB. 1980. Role of light in the regulation of chloroplast enzymes. Annual Review of Plant Physiology 31, 341–374.
-
- Buchanan BB, Balmer Y. 2005. Redox regulation: a broadening horizon. Annual Review of Plant Biology 56, 187–220. - PubMed
-
- Buchanan BB, Wolosiuk RA. 1976. Photosynthetic regulatory protein found in animal and bacterial cells. Nature 264, 669–670. - PubMed
-
- Buey RM, Galindo-Trigo S, López-Maury L, Velázquez-Campoy A, Revuelta JL, Florencio FJ, de Pereda JM, Schürmann P, Buchanan BB, Balsera M. 2017. A new member of the thioredoxin reductase family from early oxygenic photosynthetic organisms. Molecular Plant 10, 212–215. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

