Site-Specific Hyperphosphorylation Inhibits, Rather than Promotes, Tau Fibrillization, Seeding Capacity, and Its Microtubule Binding
- PMID: 31863676
- PMCID: PMC7065254
- DOI: 10.1002/anie.201913001
Site-Specific Hyperphosphorylation Inhibits, Rather than Promotes, Tau Fibrillization, Seeding Capacity, and Its Microtubule Binding
Abstract
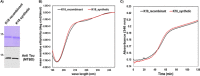
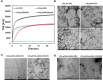
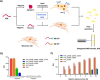
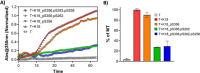
The consistent observation of phosphorylated tau in the pathology of Alzheimer's disease has contributed to the emergence of a model where hyperphosphorylation triggers both tau disassociation from microtubules and its subsequent aggregation. Herein, we applied a total chemical synthetic approach to site-specifically phosphorylate the microtubule binding repeat domain of tau (K18) at single (pS356) or multiple (pS356/pS262 and pS356/pS262/pS258) residues. We show that hyperphosphorylation of K18 inhibits 1) its aggregation in vitro, 2) its seeding activity in cells, 3) its binding to microtubules, and 4) its ability to promote microtubule polymerization. The inhibition increased with increasing the number of phosphorylated sites, with phosphorylation at S262 having the strongest effect. Our results argue against the hyperphosphorylation hypothesis and underscore the importance of revisiting the role of site-specific hyperphosphorylation in regulating tau functions in health and disease.
Keywords: aggregation; hyperphosphorylation; native state stabilization; protein modifications; tau protein.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
Hilal A. Lashuel is the founder and CSO of ND BioSciences.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

