Interplay between β-carotene and lipoprotein metabolism at the maternal-fetal barrier
- PMID: 31863969
- PMCID: PMC7302977
- DOI: 10.1016/j.bbalip.2019.158591
Interplay between β-carotene and lipoprotein metabolism at the maternal-fetal barrier
Abstract
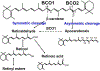
Vitamin A is an essential nutrient, critical for proper embryonic development in mammals. Both embryonic vitamin A-deficiency or -excess lead to congenital malformations or lethality in mammals, including humans. This is due to the defective transcriptional action of retinoic acid, the active form of vitamin A, that regulates in a spatial- and temporal-dependent manner the expression of genes essential for organogenesis. Thus, an adequate supply of vitamin A from the maternal circulation is vital for normal mammalian fetal development. Provitamin A carotenoids circulate in the maternal bloodstream and are available to the embryo. Of all the dietary carotenoids, β-carotene is the main vitamin A precursor, contributing at least 30% of the vitamin A intake in the industrialized countries and often constituting the sole source of retinoids (vitamin A and its derivatives) in the developing world. In humans, up to 40% of the absorbed dietary β-carotene is incorporated in its intact form in chylomicrons for distribution to other organs within the body, including the developing tissues. Here, it can serve as a source of vitamin A upon conversion into apocarotenoids by its cleavage enzymes. Given that β-carotene is carried in the bloodstream by lipoproteins, and that the placenta acquires, assembles and secretes lipoproteins, it is becoming evident that the maternal-fetal transfer of β-carotene relies on lipoprotein metabolism. Here, we will explore the current knowledge about this important biological process, the cross-talk between carotenoid and lipid metabolism in the context of the maternal-fetal transfer of this provitamin A precursor, and the mechanisms whereby β-carotene is metabolized by the developing tissues. This article is part of a Special Issue entitled Carotenoids recent advances in cell and molecular biology edited by Johannes von Lintig and Loredana Quadro.
Keywords: Lipoproteins; Microsomal triglyceride transfer protein (MTP); Retinoids; β-Carotene; β-Carotene-15,15′‑oxygenase (BCO1); β-Carotene-9′,10′‑oxygenase (BCO2).
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Microsomal triglyceride transfer protein-mediated transfer of β-carotene from donor to acceptor vesicles in vitro.Methods Enzymol. 2022;674:343-362. doi: 10.1016/bs.mie.2022.03.063. Epub 2022 Apr 29. Methods Enzymol. 2022. PMID: 36008012 Free PMC article.
-
β-Apo-10'-carotenoids Modulate Placental Microsomal Triglyceride Transfer Protein Expression and Function to Optimize Transport of Intact β-Carotene to the Embryo.J Biol Chem. 2016 Aug 26;291(35):18525-35. doi: 10.1074/jbc.M116.738336. Epub 2016 Jul 8. J Biol Chem. 2016. PMID: 27402843 Free PMC article.
-
Two carotenoid oxygenases contribute to mammalian provitamin A metabolism.J Biol Chem. 2013 Nov 22;288(47):34081-34096. doi: 10.1074/jbc.M113.501049. Epub 2013 Oct 8. J Biol Chem. 2013. PMID: 24106281 Free PMC article.
-
Maternal-fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues.Biochim Biophys Acta. 2012 Jan;1821(1):88-98. doi: 10.1016/j.bbalip.2011.05.003. Epub 2011 May 19. Biochim Biophys Acta. 2012. PMID: 21621637 Free PMC article. Review.
-
Maternal-Fetal Transfer of Vitamin A and Its Impact on Mammalian Embryonic Development.Subcell Biochem. 2020;95:27-55. doi: 10.1007/978-3-030-42282-0_2. Subcell Biochem. 2020. PMID: 32297295 Review.
Cited by
-
Genetic tuning of β-carotene oxygenase-1 activity rescues cone photoreceptor function in STRA6-deficient mice.Hum Mol Genet. 2023 Feb 19;32(5):798-809. doi: 10.1093/hmg/ddac242. Hum Mol Genet. 2023. PMID: 36150025 Free PMC article.
-
Microsomal triglyceride transfer protein-mediated transfer of β-carotene from donor to acceptor vesicles in vitro.Methods Enzymol. 2022;674:343-362. doi: 10.1016/bs.mie.2022.03.063. Epub 2022 Apr 29. Methods Enzymol. 2022. PMID: 36008012 Free PMC article.
-
Retinoic acid regulates pyruvate dehydrogenase kinase 4 (Pdk4) to modulate fuel utilization in the adult heart: Insights from wild-type and β-carotene 9',10' oxygenase knockout mice.FASEB J. 2022 Sep;36(9):e22513. doi: 10.1096/fj.202101910RR. FASEB J. 2022. PMID: 36004605 Free PMC article.
-
Home-Prepared Meal Consumption Is Associated with Healthy Food Choices in Pregnant Women Followed Up by Primary Health Care.Int J Environ Res Public Health. 2022 Dec 9;19(24):16557. doi: 10.3390/ijerph192416557. Int J Environ Res Public Health. 2022. PMID: 36554440 Free PMC article.
-
Characterization of lipoproteins in human placenta and fetal circulation as well as gestational changes in lipoprotein assembly and secretion in human and mouse placentas.Biochim Biophys Acta Mol Cell Biol Lipids. 2023 Sep;1868(9):159357. doi: 10.1016/j.bbalip.2023.159357. Epub 2023 Jun 12. Biochim Biophys Acta Mol Cell Biol Lipids. 2023. PMID: 37315736 Free PMC article.
References
-
- Hashimoto H, Uragami C, Cogdell RJ, Carotenoids and Photosynthesis, Subcell. Biochem 79 (2016) 111–139. - PubMed
-
- Kopsell DA, Kopsell DE, Carotenoids in Vegetables: Biosynthesis, Occurrence, Impacts on Human Health, and Potential for Manipulation, in: Watson RR, Preedy VR (Eds.) Bioactive Foods in Promoting Health Fruits and Vegetables, Elsevier, Inc., Place Published, 2010, pp. 645–662.
-
- Bohm F, Edge R, Truscott TG, Interactions of dietary carotenoids with singlet oxygen and free radicals: potential effects for human health, Acta Biochim. Pol 59 (2012) 27–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

