Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group
- PMID: 31864668
- PMCID: PMC11559632
- DOI: 10.1016/j.ophtha.2019.11.004
Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group
Erratum in
-
Corrigendum.Ophthalmology. 2020 Oct;127(10):1434-1435. doi: 10.1016/j.ophtha.2020.07.019. Ophthalmology. 2020. PMID: 32951677 No abstract available.
Abstract
Purpose: To establish a process to evaluate and standardize a state-of-the-art nomenclature for reporting neovascular age-related macular degeneration (AMD) data.
Design: Consensus meeting.
Participants: An international panel of retina specialists, imaging and image reading center experts, and ocular pathologists.
Methods: During several meetings organized under the auspices of the Macula Society, an international study group discussed and codified a set nomenclature framework for classifying the subtypes of neovascular AMD and associated lesion components.
Main outcome measures: A consensus classification of neovascular AMD.
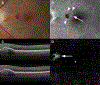
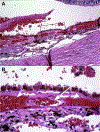
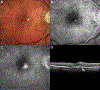
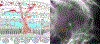
Results: The study group created a standardized working definition of AMD. The components of neovascular AMD were defined and subclassified. Disease consequences of macular neovascularization were delineated.
Conclusions: The framework of a consensus nomenclature system, a definition of AMD, and a delineation of the subtypes of neovascular AMD were developed. Establishing a uniform set of definitions will facilitate comparison of diverse patient groups and different studies. The framework presented is modified and updated readily, processes that are anticipated to occur on a periodic basis. The study group suggests that the consensus standards outlined in this article be used in future reported studies of neovascular AMD and clinical practice.
Copyright © 2019 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Figures













References
-
- Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol 1967;63(3 Suppl):1–139. - PubMed
-
- Gass JDM. Stereoscopic Atlas of Macular Diseases: A Funduscopic and Angiographic Presentation St. Louis: Mosby; 1970.
-
- Blair CJ. Geographic atrophy of the retinal pigment epithelium. A manifestation of senile macular degeneration. Arch Ophthalmol 1975;93(1):19–25. - PubMed
-
- Novais EA, Baumal CR, Sarraf D, et al. Multimodal imaging in retinal disease: a consensus definition. Ophthalmic Surg Lasers Imaging Retina. 2016;47(3):201–205. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

