Candida albicans enhances meropenem tolerance of Pseudomonas aeruginosa in a dual-species biofilm
- PMID: 31865379
- PMCID: PMC7069478
- DOI: 10.1093/jac/dkz514
Candida albicans enhances meropenem tolerance of Pseudomonas aeruginosa in a dual-species biofilm
Abstract
Background: Pseudomonas aeruginosa is an opportunistic bacterium that infects the airways of cystic fibrosis patients, surfaces of surgical and burn wounds, and indwelling medical devices. Patients are prone to secondary fungal infections, with Candida albicans being commonly co-isolated with P. aeruginosa. Both P. aeruginosa and C. albicans are able to form extensive biofilms on the surfaces of mucosa and medical devices.
Objectives: To determine whether the presence of C. albicans enhances antibiotic tolerance of P. aeruginosa in a dual-species biofilm.
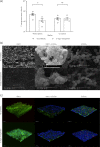
Methods: Single- and dual-species biofilms were established in microtitre plates and the survival of each species was measured following treatment with clinically relevant antibiotics. Scanning electron microscopy and confocal microscopy were used to visualize biofilm structure.
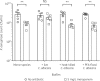
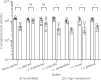
Results: C. albicans enhances P. aeruginosa biofilm tolerance to meropenem at the clinically relevant concentration of 5 mg/L. This effect is specific to biofilm cultures and is dependent upon C. albicans extracellular matrix polysaccharides, mannan and glucan, with C. albicans cells deficient in glycosylation structures not enhancing P. aeruginosa tolerance to meropenem.
Conclusions: We propose that fungal mannan and glucan secreted into the extracellular matrix of P. aeruginosa/C. albicans dual-species biofilms play a central role in enhancing P. aeruginosa tolerance to meropenem, which has direct implications for the treatment of coinfected patients.
© The Author(s) 2019. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy.
Figures



References
-
- Muthig M, Hebestreit A, Ziegler U. et al. Persistence of Candida species in the respiratory tract of cystic fibrosis patients. Med Mycol 2010; 48: 56–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

