The effect of antibiotics on clinical outcomes in immune-checkpoint blockade: a systematic review and meta-analysis of observational studies
- PMID: 31865400
- PMCID: PMC11027824
- DOI: 10.1007/s00262-019-02453-2
The effect of antibiotics on clinical outcomes in immune-checkpoint blockade: a systematic review and meta-analysis of observational studies
Abstract
Purpose: Pre-clinical and early clinical data suggests the microbiome plays an important role in oncogenesis and influences response to immune checkpoint blockade (ICB). The objective of this systematic review and meta-analysis was to determine whether antibiotics affect overall survival (OS) and progression free survival (PFS) in patients with solid malignancies treated with ICB.
Patients and methods: A systematic search of EMBASE, MEDLINE and conference proceedings was conducted for observational studies examining the effect of antibiotics on ICB. A random effects study-level meta-analysis was performed with pooling of the hazards ratio (HR) for OS and PFS. Meta-regression was used to determine the impact of the timing of antibiotic exposure on OS.
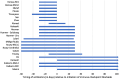
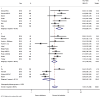
Results: 766 studies were identified, and 18 studies met the inclusion criteria. Of the 2889 patients included, 826 (28.6%) were exposed to antibiotics. The most common malignancies were lung (59%), renal cell carcinoma (RCC) or urothelial carcinoma (16.3%) and melanoma (18.7%). OS was prolonged in those without antibiotic exposure (pooled HR 1.92, 95% CI 1.37-2.68, p < 0.001). The effect of antibiotics on OS was greater in studies defining antibiotic exposure as 42 days prior to initiation of ICB (HR 3.43, 95% CI 2.29-5.14, p < 0.0001). PFS was also longer in patients who did not receive antibiotics (pooled HR 1.65, 95% CI 1.3-2.1, p < 0.0001).
Conclusion: In patients receiving ICB, OS and PFS are longer in patients who are not exposed to antibiotics. Antibiotic use in the 42 days before starting ICB appears to be most detrimental to outcome.
Keywords: Antibiotics; Cancer; Immune checkpoint blockade; Immunotherapy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, Boselli L, Routier E, Cassard L, Collins M. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–1379. doi: 10.1093/annonc/mdx108. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

