Comparison of the gamma-Pareto convolution with conventional methods of characterising metformin pharmacokinetics in dogs
- PMID: 31865474
- PMCID: PMC7040082
- DOI: 10.1007/s10928-019-09666-z
Comparison of the gamma-Pareto convolution with conventional methods of characterising metformin pharmacokinetics in dogs
Abstract
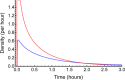
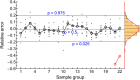
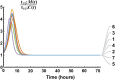
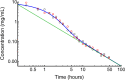
A model was developed for long term metformin tissue retention based upon temporally inclusive models of serum/plasma concentration ([Formula: see text]) having power function tails called the gamma-Pareto type I convolution (GPC) model and was contrasted with biexponential (E2) and noncompartmental (NC) metformin models. GPC models of [Formula: see text] have a peripheral venous first arrival of drug-times parameter, early [Formula: see text] peaks and very slow washouts of [Formula: see text]. The GPC, E2 and NC models were applied to a total of 148 serum samples drawn from 20 min to 72 h following bolus intravenous metformin in seven healthy mongrel dogs. The GPC model was used to calculate area under the curve (AUC), clearance ([Formula: see text]), and functions of time, f(t), for drug mass remaining (M), apparent volume of distribution ([Formula: see text]), as well as [Formula: see text] for [Formula: see text], [Formula: see text] and [Formula: see text]. The GPC models of [Formula: see text] yielded metformin [Formula: see text]-values that were 84.8% of total renal plasma flow (RPF) as estimated from meta-analysis. The GPC [Formula: see text]-values were significantly less than the corresponding NC and E2 [Formula: see text]-values of 104.7% and 123.7% of RPF, respectively. The GPC plasma/serum only model predicted 78.9% drug [Formula: see text] average urinary recovery at 72 h; similar to prior human urine drug [Formula: see text] collection results. The GPC model [Formula: see text] of [Formula: see text], [Formula: see text] and [Formula: see text], were asymptotically proportional to elapsed time, with a constant limiting [Formula: see text] ratio of M/C averaging 7.0 times, a result in keeping with prior simultaneous [Formula: see text] and urine [Formula: see text] collection studies and exhibiting a rate of apparent volume growth of [Formula: see text] that achieved limiting constant values. A simulated constant average drug mass multidosing protocol exhibited increased [Formula: see text] and [Formula: see text] with elapsing time, effects that have been observed experimentally during same-dose multidosing. The GPC heavy-tailed models explained multiple documented phenomena that were unexplained with lighter-tailed models.
Keywords: Clearance; Drug mass; Loading dose regimen; Mathematical modelling pdf; Metformin; Pharmacokinetics; Serum concentration.
Figures








References
-
- Johnston CA, Dickinson VSM, Alcorn J, Gaunt MC. Pharmacokinetics and oral bioavailability of metformin hydrochloride in healthy mixed-breed dogs. Am J Vet Res. 2017;78(10):1193–1199. - PubMed
-
- Wypij JM. Pilot study of oral metformin in cancer-bearing cats. Vet Comp Oncol. 2017;15(2):345–354. - PubMed
-
- Xie F, Ke AB, Bowers GD, Zamek-Gliszczynski MJ. Metformin’s intrinsic blood-to-plasma partition ratio (B/P): reconciling the perceived high in vivo with the in vitro equilibrium value of unity. J Pharmacol Exp Ther. 2015;354(2):225–229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

