The Intrapericardial Delivery of Extracellular Vesicles from Cardiosphere-Derived Cells Stimulates M2 Polarization during the Acute Phase of Porcine Myocardial Infarction
- PMID: 31865532
- PMCID: PMC7253530
- DOI: 10.1007/s12015-019-09926-y
The Intrapericardial Delivery of Extracellular Vesicles from Cardiosphere-Derived Cells Stimulates M2 Polarization during the Acute Phase of Porcine Myocardial Infarction
Erratum in
-
Author Correction: The Intrapericardial Delivery of Extracellular Vesicles from Cardiosphere-Derived Cells Stimulates M2 Polarization during the Acute Phase of Porcine Myocardial Infarction.Stem Cell Rev Rep. 2020 Jun;16(3):626. doi: 10.1007/s12015-020-09962-z. Stem Cell Rev Rep. 2020. PMID: 32107730 Free PMC article.
Abstract
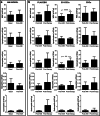
Acute myocardial infarction triggers a strong inflammatory response in the affected cardiac tissue. New therapeutic tools based on stem cell therapy may modulate the unbalanced inflammation in the damaged cardiac tissue, contributing to the resolution of this pathological condition. The main goal of this study was to analyze the immunomodulatory effects of cardiosphere-derived cells (CDCs) and their extracellular vesicles (EV-CDCs), delivered by intrapericardial administration in a clinically relevant animal model, during the initial pro-inflammatory phase of an induced myocardial infarction. This effect was assessed in peripheral blood and pericardial fluid leukocytes from infarcted animals. Additionally, cardiac functional parameters, troponin I, hematological and biochemical components were also analyzed to characterize myocardial infarction-induced changes, as well as the safety aspects of these procedures. Our preclinical study demonstrated a successful myocardial infarction induction in all animals, without any reported adverse effect related to the intrapericardial administration of CDCs or EV-CDCs. Significant changes were observed in biochemical and immunological parameters after myocardial infarction. The analysis of peripheral blood leukocytes revealed an increase of M2 monocytes in the EV-CDCs group, while no differences were reported in other lymphocyte subsets. Moreover, arginase-1 (M2-differentiation marker) was significantly increased in pericardial fluids 24 h after EV-CDCs administration. In summary, we demonstrate that, in our experimental conditions, intrapericardially administered EV-CDCs have an immunomodulatory effect on monocyte polarization, showing a beneficial effect for counteracting an unbalanced inflammatory reaction in the acute phase of myocardial infarction. These M2 monocytes have been defined as "pro-regenerative cells" with a pro-angiogenic and anti-inflammatory activity.
Keywords: Acute myocardial infarction; Cardiosphere-derived cells; Extracellular vesicles; Inflammation; Intrapericardial administration.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Dokainish H, Teo K, Zhu J, Roy A, AlHabib KF, ElSayed A, et al. Global mortality variations in patients with heart failure: Results from the international congestive heart failure (INTER-CHF) prospective cohort study. The Lancet Global Health. 2017;5(7):e665–e672. doi: 10.1016/S2214-109X(17)30196-1. - DOI - PubMed
-
- Ong S-B, Hernández-Reséndiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek X-Y, Cabrera-Fuentes HA, Hausenloy DJ. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacology & Therapeutics. 2018;186:73–87. doi: 10.1016/j.pharmthera.2018.01.001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

