Staphylococcus epidermidis Contributes to Healthy Maturation of the Nasal Microbiome by Stimulating Antimicrobial Peptide Production
- PMID: 31866425
- PMCID: PMC10988655
- DOI: 10.1016/j.chom.2019.11.003
Staphylococcus epidermidis Contributes to Healthy Maturation of the Nasal Microbiome by Stimulating Antimicrobial Peptide Production
Abstract
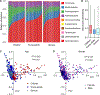
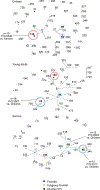
The composition of the human microbiome profoundly impacts human well-being. However, the mechanisms underlying microbiome maturation are poorly understood. The nasal microbiome is of particular importance as a source of many respiratory infections. Here, we performed a large sequencing and culture-based analysis of the human nasal microbiota from different age groups. We observed a significant decline of pathogenic bacteria before adulthood, with an increase of the commensal Staphylococcus epidermidis. In seniors, this effect was partially reversed. In vitro, many S. epidermidis isolates stimulated nasal epithelia to produce antimicrobial peptides, killing pathogenic competitors, while S. epidermidis itself proved highly resistant owing to its exceptional capacity to form biofilms. Furthermore, S. epidermidis isolates with high antimicrobial peptide-inducing and biofilm-forming capacities outcompeted pathogenic bacteria during nasal colonization in vivo. Our study identifies a pivotal role of S. epidermidis in healthy maturation of the nasal microbiome, which is achieved at least in part by symbiotic cooperation with innate host defense.
Keywords: Staphylococcus epidermidis; antimicrobial peptides; biofilm; immune evasion; innate immunity; microbiome; nose.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests
The authors declare no competing interests.
Figures




References
-
- Bosch A, de Steenhuijsen Piters WAA, van Houten MA, Chu M, Biesbroek G, Kool J, Pernet P, de Groot PCM, Eijkemans MJC, Keijser BJF, et al. (2017). Maturation of the Infant Respiratory Microbiota, Environmental Drivers, and Health Consequences. A Prospective Cohort Study. Am J Respir Crit Care Med. 196(12), 1582–1590. Published online 2017/07/01 DOI: 10.1164/rccm.201703-0554OC. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

