Targeting Alpha-Synuclein as a Therapy for Parkinson's Disease
- PMID: 31866823
- PMCID: PMC6906193
- DOI: 10.3389/fnmol.2019.00299
Targeting Alpha-Synuclein as a Therapy for Parkinson's Disease
Abstract
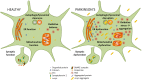
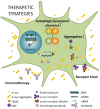
Parkinson's disease (PD) is one of the most common neurodegenerative disorders with a global burden of approximately 6.1 million patients. Alpha-synuclein has been linked to both the sporadic and familial forms of the disease. Moreover, alpha-synuclein is present in Lewy-bodies, the neuropathological hallmark of PD, and the protein and its aggregation have been widely linked to neurotoxic pathways that ultimately lead to neurodegeneration. Such pathways include autophagy/lysosomal dysregulation, synaptic dysfunction, mitochondrial disruption, and endoplasmic reticulum (ER) and oxidative stress. Alpha-synuclein has not only been shown to alter cellular pathways but also to spread between cells, causing aggregation in host cells. Therapeutic approaches will need to address several, if not all, of these angles of alpha-synuclein toxicity. Here we review the current advances in therapeutic efforts for PD that aim to produce a disease-modifying therapy by targeting the spread, production, aggregation, and degradation of alpha-synuclein. These include: receptor blocking strategies whereby putative alpha-synuclein receptors could be blocked inhibiting alpha-synuclein spread, an alpha-synuclein reduction which will decrease the amount alpha-synuclein available for aggregation and pathway disruption, the use of small molecules in order to target alpha-synuclein aggregation, immunotherapy and the increase of alpha-synuclein degradation by increasing autophagy/lysosomal flux. The research discussed here may lead to a disease-modifying therapy that tackles disease onset and progression in the future.
Keywords: Parkinson’s disease; aggregation; alpha-synuclein; fibrils; oligomers; therapy.
Copyright © 2019 Fields, Bengoa-Vergniory and Wade-Martins.
Figures
References
-
- Alarcón-Arís D., Recasens A., Galofré M., Carballo-Carbajal I., Zacchi N., Ruiz-Bronchal E., et al. . (2018). Selective α-synuclein knockdown in monoamine neurons by intranasal oligonucleotide delivery: potential therapy for Parkinson’s disease. Mol. Ther. 26, 550–567. 10.1007/s00401-009-0523-2 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

