Synaptic Clustering and Memory Formation
- PMID: 31866824
- PMCID: PMC6908852
- DOI: 10.3389/fnmol.2019.00300
Synaptic Clustering and Memory Formation
Abstract
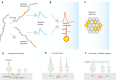
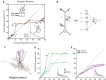
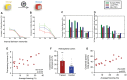
In the study of memory engrams, synaptic memory allocation is a newly emerged theme that focuses on how specific synapses are engaged in the storage of a given memory. Cumulating evidence from imaging and molecular experiments indicates that the recruitment of synapses that participate in the encoding and expression of memory is neither random nor uniform. A hallmark observation is the emergence of groups of synapses that share similar response properties and/or similar input properties and are located within a stretch of a dendritic branch. This grouping of synapses has been termed "synapse clustering" and has been shown to emerge in many different memory-related paradigms, as well as in in vitro studies. The clustering of synapses may emerge from synapses receiving similar input, or via many processes which allow for cross-talk between nearby synapses within a dendritic branch, leading to cooperative plasticity. Clustered synapses can act in concert to maximally exploit the nonlinear integration potential of the dendritic branches in which they reside. Their main contribution is to facilitate the induction of dendritic spikes and dendritic plateau potentials, which provide advanced computational and memory-related capabilities to dendrites and single neurons. This review focuses on recent evidence which investigates the role of synapse clustering in dendritic integration, sensory perception, learning, and memory as well as brain dysfunction. We also discuss recent theoretical work which explores the computational advantages provided by synapse clustering, leading to novel and revised theories of memory. As an eminent phenomenon during memory allocation, synapse clustering both shapes memory engrams and is also shaped by the parallel plasticity mechanisms upon which it relies.
Keywords: dendrites; engram; in-branch localization; memory; synaptic clustering.
Copyright © 2019 Kastellakis and Poirazi.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

