Physiological Traits for Shortening Crop Duration and Improving Productivity of Greengram (Vigna radiata L. Wilczek) Under High Temperature
- PMID: 31867025
- PMCID: PMC6904351
- DOI: 10.3389/fpls.2019.01508
Physiological Traits for Shortening Crop Duration and Improving Productivity of Greengram (Vigna radiata L. Wilczek) Under High Temperature
Abstract
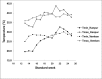
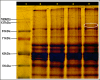
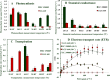
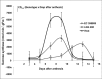
Greengram is an important protein-rich food legume crop. During the reproductive stage, high temperatures cause flower drop, induce male sterility, impair anthesis, and shortens the grain-filling period. Initially, 116 genotypes were evaluated for 3 years in two locations, and based on flowering, biomass, and yield attributes, they were grouped into four major clusters. A panel of 17 contrasting genotypes was selected for their heat tolerance in high-temperature greenhouses. The seedlings of the selected genotypes were exposed to heat shock in the range 37°C-52°C and their recovery after heat shock was assessed at 30°C. The seedlings of EC 398889 turned completely green and rejuvenated, while those of LGG 460 failed to recover, therefore, EC 398889 and LGG 460 were identified as heat-tolerant and heat-sensitive genotypes, respectively. Except for EC 398889, the remaining genotypes could not survive after heat shock. Fresh seeds of EC 398889 and LGG 460 were planted in field and pollen fertility and sucrose-synthase (SuSy) activity in grains were assessed at high temperatures. The pollen germination and SuSy activity were normal even at temperatures beyond 40°C in EC 398889 and high SuSy activity enabled faster grain filling than in LGG 460. The precise phenotyping demonstrated significant differences in the light-temperature response of photosynthesis, chlorophyll fluorescence imaging of quantum yield (Fv/Fm), and electron transport rate (ETR) between heat-tolerant (EC 398889) and heat-sensitive (LGG 460) genotypes. Molecular profiling of selected accessions showed polymorphism with 11 SSR markers and the markers CEDG147, CEDG247, and CEDG044 distinguished tolerant and sensitive groups of accessions.
Keywords: acquired thermotolerance; chlorophyll fluorescence; photosynthesis; sucrose synthase; thermo-tolerance.
Copyright © 2019 Basu, Pratap, Gupta, Sharma, Tomar and Singh.
Figures






References
-
- Ahmad A., Diwan H., Abrol Y. P. (2010). "Global climate change, stress and plant productivity," in Abiotic stress adaptation in plants: Physiological, molecular and genome foundation. Eds. Pareek A., Sopory S. K., Bohnert H. J., Govindjee (Dordrecht:Springer Science Business Media BV; ), 503–521. 10.1007/978-90-481-3112-9_23 - DOI
-
- Anderson A. J., Sonali P. R. (2004). Protein aggregation, radicals scavenging capacity, and stability of hydrogen peroxide defence system in heat stressed Vinca and sweet pea leaves. J. Am. Soc. Hortic. Sci 129, 54–59. 10.21273/JASHS.129.1.0054 - DOI
-
- Arunyanark A., Jogloy S., Vorasoot N., Akkasaeng C., Kesmala T., Patanothai A. (2009). Stability of relationship between chlorophyll density and soil plant analysis development chlorophyll meter readings in peanut across different drought stress conditions. Asian J. Plant Sci. 8, 102–110. 10.3923/ajps.2009.102.110 - DOI
LinkOut - more resources
Full Text Sources

