Downstream Processing of Chlamydomonas reinhardtii TN72 for Recombinant Protein Recovery
- PMID: 31867315
- PMCID: PMC6908742
- DOI: 10.3389/fbioe.2019.00383
Downstream Processing of Chlamydomonas reinhardtii TN72 for Recombinant Protein Recovery
Abstract
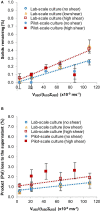
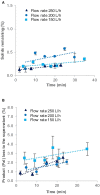
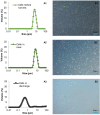
The green microalga Chlamydomonas reinhardtii is under development as a production host for recombinant proteins and whole-cell therapeutics. In particular, the cell wall-reduced strain TN72 is used as a model organism for protein expression and algal synthetic biology. However, the bioprocessing characteristics of TN72 and other C. reinhardtii strains have yet to be examined. Here we use a TN72 strain expressing a protein-based antibiotic (Pal) to study the scale-up of cell harvest and product recovery. Cell harvest was examined with 100L cultures in two intermittent-discharge continuous-flow disc-stack centrifuges at flow rates of 150-250 L.h-1, as well as with an ultra scale-down (USD) mimic of the centrifuges. Solids recovery exceeded 99.5% and the loss of product to the supernatant was below 2-3%. TN72 is intact following the high shear conditions of the feed zone, however discharge from both disc-stack centrifuges resulted in full cell breakage and in the case of Pal, partial degradation in the subsequent hours. We demonstrated that shake flask cultivation and the USD centrifuge technique can be used to predict the pilot-scale clarification efficiency and product release at the centrifuge inlet for TN72, but not the cell breakage on discharge. This study outlines a number of challenges for scale-up of recombinant protein production in the microalgal host in particular for whole cell therapeutics, but also opportunities for the bioprocessing of intracellular products from TN72.
Keywords: algae; disc-stack centrifugation; downstream processing; endolysin; scale-up; ultra scale-down.
Copyright © 2019 Stoffels, Finlan, Mannall, Purton and Parker.
Figures



Similar articles
-
Prediction and verification of centrifugal dewatering of P. pastoris fermentation cultures using an ultra scale-down approach.Biotechnol Bioeng. 2012 Aug;109(8):2039-47. doi: 10.1002/bit.24478. Epub 2012 Mar 22. Biotechnol Bioeng. 2012. PMID: 22442107
-
Ultra scale-down prediction using microwell technology of the industrial scale clarification characteristics by centrifugation of mammalian cell broths.Biotechnol Bioeng. 2009 Oct 1;104(2):321-31. doi: 10.1002/bit.22393. Biotechnol Bioeng. 2009. PMID: 19507199
-
Ultra scale-down approaches for clarification of mammalian cell culture broths in disc-stack centrifuges.Biotechnol Prog. 2009 Nov-Dec;25(6):1709-16. doi: 10.1002/btpr.275. Biotechnol Prog. 2009. PMID: 19768799
-
Outlook in the application of Chlamydomonas reinhardtii chloroplast as a platform for recombinant protein production.Biotechnol Genet Eng Rev. 2016 Apr-Oct;32(1-2):92-106. doi: 10.1080/02648725.2017.1307673. Epub 2017 Mar 30. Biotechnol Genet Eng Rev. 2016. PMID: 28359189 Review.
-
Manufacturing of Proteins and Antibodies: Chapter Downstream Processing Technologies.Adv Biochem Eng Biotechnol. 2018;165:95-114. doi: 10.1007/10_2016_54. Adv Biochem Eng Biotechnol. 2018. PMID: 28776064 Review.
Cited by
-
Key Proteomics Tools for Fundamental and Applied Microalgal Research.Proteomes. 2024 Apr 4;12(2):13. doi: 10.3390/proteomes12020013. Proteomes. 2024. PMID: 38651372 Free PMC article. Review.
-
Accelerating Chloroplast Engineering: A New System for Rapid Generation of Marker-Free Transplastomic Lines of Chlamydomonas reinhardtii.Microorganisms. 2023 Jul 31;11(8):1967. doi: 10.3390/microorganisms11081967. Microorganisms. 2023. PMID: 37630526 Free PMC article.
-
Multigenic engineering of the chloroplast genome in the green alga Chlamydomonas reinhardtii.Microbiology (Reading). 2020 Jun;166(6):510-515. doi: 10.1099/mic.0.000910. Microbiology (Reading). 2020. PMID: 32250732 Free PMC article.
-
Spray Drying Is a Viable Technology for the Preservation of Recombinant Proteins in Microalgae.Microorganisms. 2023 Feb 17;11(2):512. doi: 10.3390/microorganisms11020512. Microorganisms. 2023. PMID: 36838478 Free PMC article.
-
Harnessing the Algal Chloroplast for Heterologous Protein Production.Microorganisms. 2022 Mar 30;10(4):743. doi: 10.3390/microorganisms10040743. Microorganisms. 2022. PMID: 35456794 Free PMC article. Review.
References
-
- Ambler C. M. (1959). The theory of scaling up laboratory data for the sedimentation type centrifuge. J. Biochem. Microbiol. Technol. Eng. 1, 185–205. 10.1002/jbmte.390010206 - DOI
-
- Aucamp J. P., Davies R., Hallet D., Weiss A., Titchener-Hooker N. J. (2014). Integration of host strain bioengineering and bioprocess development using ultra-scale down studies to select the optimum combination: an antibody fragment primary recovery case study. Biotechnol. Bioeng. 111, 1971–1981. 10.1002/bit.25259 - DOI - PMC - PubMed
-
- Borowitzka M. A., Vonshak A. (2017). Scaling up microalgal cultures to commercial scale. Eur. J. Phycol. 52, 407–418. 10.1080/09670262.2017.1365177 - DOI
LinkOut - more resources
Full Text Sources

