Glycosphingolipids and Infection. Potential New Therapeutic Avenues
- PMID: 31867330
- PMCID: PMC6908816
- DOI: 10.3389/fcell.2019.00324
Glycosphingolipids and Infection. Potential New Therapeutic Avenues
Abstract
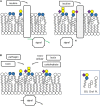
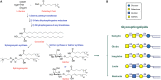
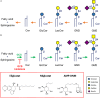
Glycosphingolipids (GSLs), the main topic of this review, are a subclass of sphingolipids. With their glycans exposed to the extracellular space, glycosphingolipids are ubiquitous components of the plasma membrane of cells. GSLs are implicated in a variety of biological processes including specific infections. Several pathogens use GSLs at the surface of host cells as binding receptors. In addition, lipid-rafts in the plasma membrane of host cells may act as platform for signaling the presence of pathogens. Relatively common in man are inherited deficiencies in lysosomal glycosidases involved in the turnover of GSLs. The associated storage disorders (glycosphingolipidoses) show lysosomal accumulation of substrate(s) of the deficient enzyme. In recent years compounds have been identified that allow modulation of GSLs levels in cells. Some of these agents are well tolerated and already used to treat lysosomal glycosphingolipidoses. This review summarizes present knowledge on the role of GSLs in infection and subsequent immune response. It concludes with the thought to apply glycosphingolipid-lowering agents to prevent and/or combat infections.
Keywords: glucosylceramide; glycosidase; glycosphingolipid; glycosyltransferase; infection; lysosome.
Copyright © 2019 Aerts, Artola, van Eijk, Ferraz and Boot.
Figures

References
-
- Aerts J. M., van Breemen M. J., Bussink A. P., Ghauharali K., Sprenger R., Boot R. G., et al. (2008). Biomarkers for lysosomal storage disorders: identification and application as exemplified by chitotriosidase in Gaucher disease. Acta Paediatr. 97 7–14. 10.1111/j.1651-2227.2007.00641.x - DOI - PubMed
-
- Ashe K. M., Bangari D., Li L., Cabrera-Salazar M. A., Bercury S. D., Nietupski J. B., et al. (2011). Iminosugar-based inhibitors of glucosylceramide synthase increase brain glycosphingolipids and survival in a mouse model of Sandhoff disease. PLoS One 6:e21758. 10.1371/journal.pone.0021758 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

