Noncytotoxic Pyrrolobenzodiazepine-Ciprofloxacin Conjugate with Activity against Mycobacterium tuberculosis
- PMID: 31867477
- PMCID: PMC6921268
- DOI: 10.1021/acsomega.9b00834
Noncytotoxic Pyrrolobenzodiazepine-Ciprofloxacin Conjugate with Activity against Mycobacterium tuberculosis
Abstract
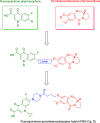
The development of new antitubercular agents for the treatment of infections caused by multidrug-resistant (MDR) Mycobacterium tuberculosis is an urgent priority. Pyrrolobenzodiazepines (PBDs) are a promising class of antibacterial agents that were initially discovered and isolated from a range of Streptomyces species. Recently, C8-linked PBD monomers have been shown to work by inhibiting DNA gyrase and have demonstrated activity against M. tuberculosis. However, both PBD monomers and dimers are toxic to eukaryotic cells, limiting their development as antibacterial agents. To eliminate the toxicity associated with PBDs and explore the effect of C8-modification with a known antibacterial agent with the same mechanism of action (i.e., ciprofloxacin, a gyrase inhibitor), we synthesized a C8-linked PBD-ciprofloxacin (PBD-CIP, 3) hybrid. The hybrid compound displayed minimum inhibitory concentration values of 0.4 or 2.1 μg/mL against drug-sensitive and drug-resistant M. tuberculosis strains, respectively. A molecular modeling study showed good interaction of compound 3 with wild-type M. tuberculosis DNA gyrase, suggesting gyrase inhibition as a possible mechanism of action. Compound 3 is a nontoxic combination hybrid that can be utilized as a new scaffold and further optimized to develop new antitubercular agents.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Global Tuberculosis Report 2018; World Health Organization, 2018.
Grants and funding
LinkOut - more resources
Full Text Sources

