Strategy on Persisting in Distinct Activity of Plasmon-Activated Water
- PMID: 31867513
- PMCID: PMC6921674
- DOI: 10.1021/acsomega.9b02627
Strategy on Persisting in Distinct Activity of Plasmon-Activated Water
Abstract
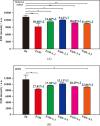
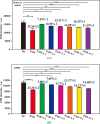
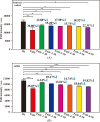
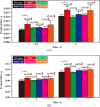
The innovative plasmon-activated water (PAW) with reduced hydrogen bonds exhibits intrinsically distinct properties at room temperature, which are significantly different from the properties of untreated conventional deionized (DI) water. Examples of this are their ability to scavenge free radicals and higher vapor pressure. However, distinct properties of energetic PAW decay within the day after its creation in a metastable liquid state. In this work, we report a facile method for persisting its distinct activities by letting as-prepared PAW be quickly frozen in liquid nitrogen and letting the frozen PAW (for one month before further measurements) be quickly melted to room temperature in a warm-water bath (called treated PAW). Experimental results indicate that the activity of the higher evaporation rate of treated PAW compared to DI water can be maintained ca. 90% of magnitude, as compared to the as-prepared PAW. Also, its abilities to scavenge free hydroxyl and 2,2-diphenyl-1-picrylhydrazyl radicals can be maintained at ca. 70 and 80% of magnitudes, respectively. Moreover, this strategy of quickly freezing and melting treatments to PAW on persisting in distinct activity of PAW is effective in oxygen evolution reactions. This promises the stored energy and the distinct property of created liquid PAW being available in water-related fields after long-term storage.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Therapeutics for Inflammatory-Related Diseases Based on Plasmon-Activated Water: A Review.Int J Mol Sci. 2018 May 28;19(6):1589. doi: 10.3390/ijms19061589. Int J Mol Sci. 2018. PMID: 29843406 Free PMC article. Review.
-
Plasmon-Activated Water can Prolong Existing Sea-Ice Habitats to Potentially Save Polar Bears.Sci Rep. 2019 Jul 18;9(1):10398. doi: 10.1038/s41598-019-46867-5. Sci Rep. 2019. PMID: 31320695 Free PMC article.
-
New solar energy-storage resource of plasmon-activated water solution with higher chemical potential.Sci Rep. 2020 Nov 30;10(1):20868. doi: 10.1038/s41598-020-77815-3. Sci Rep. 2020. PMID: 33257784 Free PMC article.
-
Active and stable liquid water innovatively prepared using resonantly illuminated gold nanoparticles.ACS Nano. 2014 Mar 25;8(3):2704-13. doi: 10.1021/nn406403c. Epub 2014 Feb 19. ACS Nano. 2014. PMID: 24533852
-
Review on formation of cold plasma activated water (PAW) and the applications in food and agriculture.Food Res Int. 2022 Jul;157:111246. doi: 10.1016/j.foodres.2022.111246. Epub 2022 Apr 21. Food Res Int. 2022. PMID: 35761559 Review.
Cited by
-
Functional Plasmon-Activated Water Increases Akkermansia muciniphila Abundance in Gut Microbiota to Ameliorate Inflammatory Bowel Disease.Int J Mol Sci. 2022 Sep 28;23(19):11422. doi: 10.3390/ijms231911422. Int J Mol Sci. 2022. PMID: 36232724 Free PMC article.
References
-
- Jin J.; Goddard W. A. III. Mechanisms Underlying The Mpemba Effect in Water from Molecular Dynamics Simulations. J. Phys. Chem. C 2015, 119, 2622–2629. 10.1021/jp511752n. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
