Synthesis and Characterization of a Click-Assembled 18-Atom Macrocycle That Displays Selective AXL Kinase Inhibitory Activity
- PMID: 31867559
- PMCID: PMC6921642
- DOI: 10.1021/acsomega.9b03525
Synthesis and Characterization of a Click-Assembled 18-Atom Macrocycle That Displays Selective AXL Kinase Inhibitory Activity
Abstract
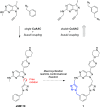
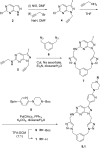
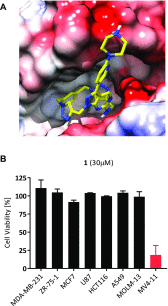
A novel macrocyclic construct consisting of a pyrazolopyrimidine scaffold concatenated to a benzene ring through two triazoles has been developed to investigate uncharted chemical space with bioactive potential. The 18-atom macrocycle was assembled via a double copper-catalyzed alkyne-azide cycloaddition (CuAAC) reaction between 1,3-bis(azidomethyl)benzene and a bis-propargylated pyrazolo[3,4-d]pyrimidine core. The resulting macrocycle was functionalized further into a multicyclic analog that displays selective inhibitory activity against the receptor tyrosine kinase AXL.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
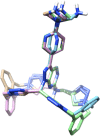

Similar articles
-
Visible-Light-Mediated Click Chemistry for Highly Regioselective Azide-Alkyne Cycloaddition by a Photoredox Electron-Transfer Strategy.Chemistry. 2020 May 4;26(25):5694-5700. doi: 10.1002/chem.202000252. Epub 2020 Apr 17. Chemistry. 2020. PMID: 31953964
-
Synthesis of bi- and bis-1,2,3-triazoles by copper-catalyzed Huisgen cycloaddition: A family of valuable products by click chemistry.Beilstein J Org Chem. 2015 Dec 11;11:2557-76. doi: 10.3762/bjoc.11.276. eCollection 2015. Beilstein J Org Chem. 2015. PMID: 26734102 Free PMC article. Review.
-
Regioselective synthesis of multisubstituted 1,2,3-triazoles: moving beyond the copper-catalyzed azide-alkyne cycloaddition.Chem Commun (Camb). 2016 Dec 6;52(99):14188-14199. doi: 10.1039/c6cc06194j. Chem Commun (Camb). 2016. PMID: 27711308
-
Catalytic "active-metal" template synthesis of [2]rotaxanes, [3]rotaxanes, and molecular shuttles, and some observations on the mechanism of the cu(i)-catalyzed azide-alkyne 1,3-cycloaddition.J Am Chem Soc. 2007 Oct 3;129(39):11950-63. doi: 10.1021/ja073513f. Epub 2007 Sep 11. J Am Chem Soc. 2007. PMID: 17845039
-
Copper-Catalyzed Azide-Alkyne Cycloaddition (CuAAC)-Mediated Macrocyclization of Peptides: Impact on Conformation and Biological Activity.Curr Top Med Chem. 2018;18(7):591-610. doi: 10.2174/1568026618666180518095755. Curr Top Med Chem. 2018. PMID: 29773065 Review.
Cited by
-
Discovery of pyrazolopyrimidines that selectively inhibit CSF-1R kinase by iterative design, synthesis and screening against glioblastoma cells.RSC Med Chem. 2023 Oct 11;14(12):2611-2624. doi: 10.1039/d3md00454f. eCollection 2023 Dec 13. RSC Med Chem. 2023. PMID: 38099057 Free PMC article.
-
Recent developments in anticancer kinase inhibitors based on the pyrazolo[3,4-d]pyrimidine scaffold.RSC Med Chem. 2020 Sep 8;11(10):1112-1135. doi: 10.1039/d0md00227e. eCollection 2020 Oct 1. RSC Med Chem. 2020. PMID: 33479617 Free PMC article. Review.
-
Ligand-centred phenotype-driven development of potent kinase inhibitors against oesophageal cancer.RSC Med Chem. 2024 Oct 15;16(1):379-91. doi: 10.1039/d4md00579a. Online ahead of print. RSC Med Chem. 2024. PMID: 39493221 Free PMC article.
References
-
- Fraser C.; Carragher N. O.; Unciti-Broceta A. eCF309: a Potent, Selective and Cell-Permeable mTOR Inhibitor. Med. Chem. Commun. 2016, 7, 471–477. 10.1039/C5MD00493D. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

