Targeted Nanoparticle Binding to Hydroxyapatite in a High Serum Environment for Early Detection of Heart Disease
- PMID: 31867573
- PMCID: PMC6924636
- DOI: 10.1021/acsanm.8b01099
Targeted Nanoparticle Binding to Hydroxyapatite in a High Serum Environment for Early Detection of Heart Disease
Abstract
The impact of the protein-rich in vivo environment on targeted binding of functionalized nanoparticles has been an active field of research over the past several years. Current research aims at better understanding the nature of the protein corona and how it may be possible for targeted binding to occur even in the presence of serum. Much of the current research focuses on nanoparticles targeted to particular cell receptors or features with the aim of cellular uptake. However, similar research has not been performed on nanoparticles that are targeted to non-protein disease features, such as hydroxyapatite (HA). HA is a crystalline calcium-phosphate mineral that is present in large quantities in bone, and in smaller quantities in diseased cardiovascular tissue in cases of atherosclerosis or various stenoses. Our work aims to gain a better understanding of the behavior of PEGylated, peptide-coated superparamagnetic iron oxide nanoparticles (SPIONs) in a biologically-relevant high-protein environment (50% serum). We first determined that specific binding to HA occurs at significantly higher rates than non-specific binding in the absence of serum protein. We then examined nanoparticle interactions with serum proteins, including determination of the relative quantities of protein in the hard vs. soft protein corona. Finally, we examined specific and non-specific binding of targeted SPIONs in 50% serum, and determined that targeted binding may still occur with significant (p < 0.05) selectivity. We hypothesize that this may be because the nature of the binding interactions between the peptides and the HA are, by definition, less specific than the protein-protein interactions required for nanoparticles to bind to specific cells or cell features. These results suggest that these targeted SPIONs may be further developed for use in early detection of heart diseases such as atherosclerosis and aortic stenosis.
Keywords: iron oxide nanoparticles; non-specific binding; protein corona; serum; targeted nanoparticles.
Figures
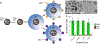

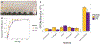
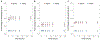
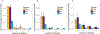
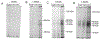
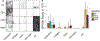
Similar articles
-
Bio-identity and fate of albumin-coated SPIONs evaluated in cells and by the C. elegans model.Acta Biomater. 2016 Oct 1;43:348-357. doi: 10.1016/j.actbio.2016.07.024. Epub 2016 Jul 15. Acta Biomater. 2016. PMID: 27427227
-
Proteomics Analysis Reveals Distinct Corona Composition on Magnetic Nanoparticles with Different Surface Coatings: Implications for Interactions with Primary Human Macrophages.PLoS One. 2015 Oct 7;10(10):e0129008. doi: 10.1371/journal.pone.0129008. eCollection 2015. PLoS One. 2015. PMID: 26444829 Free PMC article.
-
Promoting the Delivery of Nanoparticles to Atherosclerotic Plaques by DNA Coating.ACS Appl Mater Interfaces. 2019 Apr 17;11(15):13888-13904. doi: 10.1021/acsami.8b17928. Epub 2018 Dec 5. ACS Appl Mater Interfaces. 2019. PMID: 30516979
-
Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: opportunities and challenges.Expert Opin Drug Deliv. 2014 Sep;11(9):1449-70. doi: 10.1517/17425247.2014.924501. Epub 2014 May 29. Expert Opin Drug Deliv. 2014. PMID: 24870351 Review.
-
Nanoparticle-Protein Interactions: Therapeutic Approaches and Supramolecular Chemistry.Acc Chem Res. 2017 Jun 20;50(6):1383-1390. doi: 10.1021/acs.accounts.7b00051. Epub 2017 May 8. Acc Chem Res. 2017. PMID: 28480714 Review.
Cited by
-
Hybrid Hydroxyapatite-Metal Complex Materials Derived from Amino Acids and Nucleobases.Molecules. 2024 Sep 20;29(18):4479. doi: 10.3390/molecules29184479. Molecules. 2024. PMID: 39339474 Free PMC article. Review.
-
Calcium-binding nanoparticles for vascular disease.Regen Eng Transl Med. 2019 Mar;5(1):74-85. Epub 2018 Oct 23. Regen Eng Transl Med. 2019. PMID: 31106257 Free PMC article.
-
The Effect of Doping on the Electrical and Dielectric Properties of Hydroxyapatite for Medical Applications: From Powders to Thin Films.Materials (Basel). 2024 Jan 28;17(3):640. doi: 10.3390/ma17030640. Materials (Basel). 2024. PMID: 38591446 Free PMC article. Review.
-
Nanocarriers of shRNA-Runx2 directed to collagen IV as a nanotherapeutic system to target calcific aortic valve disease.Mater Today Bio. 2023 Mar 28;20:100620. doi: 10.1016/j.mtbio.2023.100620. eCollection 2023 Jun. Mater Today Bio. 2023. PMID: 37063777 Free PMC article.
-
Substituted hydroxyapatite coatings of bone implants.J Mater Chem B. 2020 Mar 4;8(9):1781-1800. doi: 10.1039/c9tb02710f. J Mater Chem B. 2020. PMID: 32065184 Free PMC article. Review.
References
-
- Gao X; Cui Y; Levenson RM; Chung LWK; Nie S In Vivo Cancer Targeting and Imaging with Semiconductor Quantum Dots. Nat. Biotechnol 2004, 22 (8), 969. - PubMed
-
- Sardan M; Eren ED; Ozdemir A; Tekinay AB; Guler MO Bioactive Peptide Functionalized Superparamagnetic Iron Oxide Nanoparticles (SPIONs) for Targeted Imaging with MRI. In 2015 5th International Workshop on Magnetic Particle Imaging (IWMPI); 2015; pp 1–1.
-
- Mu K; Zhang S; Ai T; Jiang J; Yao Y; Jiang L; Zhou Q; Xiang H; Zhu Y; Yang X; Zhu W Monoclonal Antibody-Conjugated Superparamagnetic Iron Oxide Nanoparticles for Imaging of Epidermal Growth Factor Receptor-Targeted Cells and Gliomas. Mol. Imaging 2015, 14. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
