Loss of Preexisting Immunological Memory Among Human Immunodeficiency Virus-Infected Women Despite Immune Reconstitution With Antiretroviral Therapy
- PMID: 31867597
- PMCID: PMC7323495
- DOI: 10.1093/infdis/jiz678
Loss of Preexisting Immunological Memory Among Human Immunodeficiency Virus-Infected Women Despite Immune Reconstitution With Antiretroviral Therapy
Abstract
Background: It is unclear whether human immunodeficiency virus (HIV) infection results in permanent loss of T-cell memory or if it affects preexisting antibodies to childhood vaccinations or infections.
Methods: We conducted a matched cohort study involving 50 pairs of HIV-infected and HIV-uninfected women. Total memory T-cell responses were measured after anti-CD3 or vaccinia virus (VV) stimulation to measure T cells elicited after childhood smallpox vaccination. VV-specific antibodies were measured by means of enzyme-linked immunosorbent assay (ELISA).
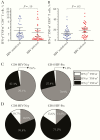
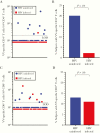
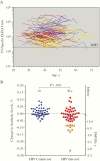
Results: There was no difference between HIV-infected and HIV-uninfected study participants in terms of CD4+ T-cell responses after anti-CD3 stimulation (P = .19) although HIV-infected participants had significantly higher CD8+ T-cell responses (P = .03). In contrast, there was a significant loss in VV-specific CD4+ T-cell memory among HIV-infected participants (P = .04) whereas antiviral CD8+ T-cell memory remained intact (P > .99). VV-specific antibodies were maintained indefinitely among HIV-uninfected participants (half-life, infinity; 95% confidence interval, 309 years to infinity) but declined rapidly among HIV-infected participants (half-life; 39 years; 24-108 years; P = .001).
Conclusions: Despite antiretroviral therapy-associated improvement in CD4+ T-cell counts (nadir, <200/μL; >350/μL after antiretroviral therapy), antigen-specific CD4+ T-cell memory to vaccinations or infections that occurred before HIV infection did not recover after immune reconstitution, and a previously unrealized decline in preexisting antibody responses was observed.
Keywords: ART; HIV; antiretroviral therapy; immunological memory; smallpox; vaccination.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Early Antiretroviral Therapy May Preserve Vaccine Responses in Human Immunodeficiency Virus-Infected Patients by Preventing Damage to Long-Lived Plasma Cells.J Infect Dis. 2020 Jun 29;222(2):176-179. doi: 10.1093/infdis/jiz679. J Infect Dis. 2020. PMID: 31867631 No abstract available.
References
-
- Kaplan JE, Hanson D, Dworkin MS, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis 2000; 30(suppl 1):S5–14. - PubMed
-
- Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol 2012; 24:501–6. - PubMed
-
- Wendland T, Furrer H, Vernazza PL, et al. HAART in HIV-infected patients: restoration of antigen-specific CD4 T-cell responses in vitro is correlated with CD4 memory T-cell reconstitution, whereas improvement in delayed type hypersensitivity is related to a decrease in viraemia. AIDS 1999; 13:1857–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AI027767/AI/NIAID NIH HHS/United States
- P51 OD011092/OD/NIH HHS/United States
- U01 AI031834/AI/NIAID NIH HHS/United States
- U01 AI035004/AI/NIAID NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- P30 AI050409/AI/NIAID NIH HHS/United States
- U01 AI034993/AI/NIAID NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- KL2 TR001432/TR/NCATS NIH HHS/United States
- U01 AI034989/AI/NIAID NIH HHS/United States
- U01 AI034994/AI/NIAID NIH HHS/United States
- U19 AI109948/AI/NIAID NIH HHS/United States
- R21 AG059505/AG/NIA NIH HHS/United States
- U01 HL146204/HL/NHLBI NIH HHS/United States
- U01 HL146202/HL/NHLBI NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- U01 HD032632/HD/NICHD NIH HHS/United States
- U01 AI042590/AI/NIAID NIH HHS/United States

