Examining Age as a Potential Moderator of Response to Reduced Nicotine Content Cigarettes in Vulnerable Populations
- PMID: 31867655
- PMCID: PMC6939764
- DOI: 10.1093/ntr/ntz134
Examining Age as a Potential Moderator of Response to Reduced Nicotine Content Cigarettes in Vulnerable Populations
Abstract
Introduction: Young adults (aged 18-24 years) have a higher smoking prevalence than younger and older age groups and young adulthood is an important developmental period during which long-term behavior patterns like cigarette smoking are established. The aim of the current study was to examine how young adult smokers with additional vulnerabilities to smoking respond to reduced nicotine content cigarettes.
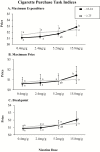
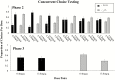
Methods: This is a secondary analysis of a double-blind, within-subject experiment conducted with 169 cigarette smokers recruited from populations with comorbid psychiatric conditions or socioeconomic disadvantage assessing acute effects of research cigarettes varying in nicotine content (0.4, 2.4, 5.2, 15.8 mg/g). Participants were dichotomized by chronological age (18-24 vs. ≥25 years). Across 14 laboratory sessions effects of nicotine content were examined on measures of relative reinforcing efficacy (Cigarette Purchase Task [CPT] and Concurrent Choice testing), subjective effects, craving/withdrawal, and smoking topography. Repeated measures analysis of variances were used to examine potential moderating effects of age.
Results: Young adults exhibited lower demand for reduced nicotine content cigarettes than older adults across three of five CPT indices (ps < .05). No differences by age were observed on other measures of reinforcing efficacy, subjective effects, craving/withdrawal, or smoking topography where effects generally decreased as an orderly function of decreasing nicotine content (ps <.05).
Conclusion: Overall, these findings suggest that reducing the nicotine content of cigarettes would decrease the addiction potential of cigarette smoking in young adult smokers as much or perhaps more than older adult smokers from populations at increased vulnerability to smoking, addiction, and smoking-related health consequences.
Implications: Reducing the nicotine content in cigarettes to lower addiction potential of smoking has been proposed as a means to improve overall population health. It is imperative to examine how young adults may respond to a nicotine reduction policy. We saw minimal evidence that age moderates acute response and where there was evidence it was in the direction of reduced nicotine content cigarettes having less addictive potential among young versus older adults (eg, steeper decreases in demand for very low nicotine content cigarettes among young versus older adults). Overall, a nicotine reduction policy has the potential to reduce smoking across age groups.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco.
Figures
References
-
- The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. https://www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf
-
- The Health Consequences of Smoking: Nicotine Addiction: A Report of the Surgeon General. Washington, DC: US. Department of Health and Human Services (DHHS Publication No. CDC 90-8416), U.S. Government Printing Office. 1988;https://profiles.nlm.nih.gov/ps/access/nnbbzd.pdf.
-
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331(2):123–125. - PubMed
-
- Tobacco Product Standard for Nicotine Level of Combusted Cigarettes: A Proposed Rule by the Food and Drug Administration on 3/16/2018. Washington, DC: Food and Drug Administration, HHS; 3/16/2018. https://www.federalregister.gov/documents/2018/03/16/2018-05345/tobacco-...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

