Development, calibration, and validation of a novel human ventricular myocyte model in health, disease, and drug block
- PMID: 31868580
- PMCID: PMC6970534
- DOI: 10.7554/eLife.48890
Development, calibration, and validation of a novel human ventricular myocyte model in health, disease, and drug block
Abstract
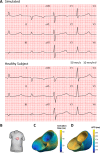
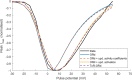
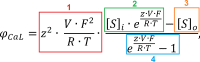
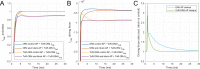
Human-based modelling and simulations are becoming ubiquitous in biomedical science due to their ability to augment experimental and clinical investigations. Cardiac electrophysiology is one of the most advanced areas, with cardiac modelling and simulation being considered for virtual testing of pharmacological therapies and medical devices. Current models present inconsistencies with experimental data, which limit further progress. In this study, we present the design, development, calibration and independent validation of a human-based ventricular model (ToR-ORd) for simulations of electrophysiology and excitation-contraction coupling, from ionic to whole-organ dynamics, including the electrocardiogram. Validation based on substantial multiscale simulations supports the credibility of the ToR-ORd model under healthy and key disease conditions, as well as drug blockade. In addition, the process uncovers new theoretical insights into the biophysical properties of the L-type calcium current, which are critical for sodium and calcium dynamics. These insights enable the reformulation of L-type calcium current, as well as replacement of the hERG current model.
Keywords: calibration validation; cell biology; computational biology; computer simulations; heart; human; systems biology; ventricular myocyte.
Plain language summary
Decades of intensive experimental and clinical research have revealed much about how the human heart works. Though incomplete, this knowledge has been used to construct computer models that represent the activity of this organ as a whole, and of its individual chambers (the atria and ventricles), tissues and cells. Such models have been used to better understand life-threatening irregular heartbeats; they are also beginning to be used to guide decisions about the treatment of patients and the development of new drugs by the pharmaceutical industry. Yet existing computer models of the electrical activity of the human heart are sometimes inconsistent with experimental data. This problem led Tomek et al. to try to create a new model that was consistent with established biophysical knowledge and experimental data for a wide range of conditions including disease and drug action. Tomek et al. designed a strategy that explicitly separated the construction and validation of a model that could recreate the electrical activity of the ventricles in a human heart. This model was able to integrate and explain a wide range of properties of both healthy and diseased hearts, including their response to different drugs. The development of the model also uncovered and resolved theoretical inconsistencies that have been present in almost all models of the heart from the last 25 years. Tomek et al. hope that their new human heart model will enable more basic, translational and clinical research into a range of heart diseases and accelerate the development of new therapies.
© 2019, Tomek et al.
Conflict of interest statement
JT, AB, EP, XZ, AM, OB, CB, SS, AS, LV, AV, BR No competing interests declared
Figures

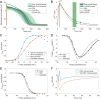
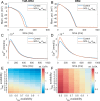

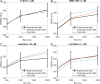


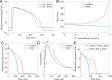



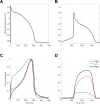
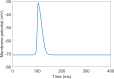

References
-
- Bousseljot R, Kreiseler D, Schnabel A. Nutzung der EKG-Signaldatenbank CARDIODAT der PTB über das internet. Biomedizinische Technik. 1995;40:317–318. doi: 10.1515/bmte.1995.40.s1.317. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- Machine learning research award/Amazon Web Services/International
- 100246/Z/12/Z/Wellcome/International
- NC/P001076/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- 214290/Z/18/Z/Wellcome/International
- 675451/European Commission/International
- FS/17/22/32644/BHF_/British Heart Foundation/United Kingdom
- TransQST project (Innovative Medicines Initiative 2 Joint Undertaking 116030)/Horizon 2020/International
- 2017174226/Partnership for Advanced Computing in Europe AISBL/International
- PRACE (2017174226)/UK National Supercomputing/International
- Archer RAP award (322 00180)/UK National Supercomputing/International
- RE/13/1/30181/BHF Centre of Research Excellence, Oxford/International
- TransQST project (Innovative Medicines Initiative 2 Joint Undertaking 116030)/European Federation of Pharmaceutical Industries and Associations/International
- 116030/TransQST/International
LinkOut - more resources
Full Text Sources

