Pharmacodynamics of Glyburide, Metformin, and Glyburide/Metformin Combination Therapy in the Treatment of Gestational Diabetes Mellitus
- PMID: 31869430
- PMCID: PMC7561209
- DOI: 10.1002/cpt.1749
Pharmacodynamics of Glyburide, Metformin, and Glyburide/Metformin Combination Therapy in the Treatment of Gestational Diabetes Mellitus
Abstract
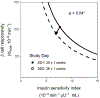
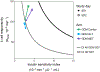
In gestational diabetes mellitus (GDM), women are unable to compensate for the increased insulin resistance during pregnancy. Data are limited regarding the pharmacodynamic effects of metformin and glyburide during pregnancy. This study characterized insulin sensitivity (SI), β-cell responsivity, and disposition index (DI) in women with GDM utilizing a mixed-meal tolerance test (MMTT) before and during treatment with glyburide monotherapy (GLY, n = 38), metformin monotherapy (MET, n = 34), or GLY and MET combination therapy (COMBO; n = 36). GLY significantly decreased dynamic β-cell responsivity (31%). MET and COMBO significantly increased SI (121% and 83%, respectively). Whereas GLY, MET, and COMBO improved DI, metformin (MET and COMBO) demonstrated a larger increase in DI (P = 0.05) and a larger decrease in MMTT peak glucose concentrations (P = 0.03) than subjects taking only GLY. Maximizing SI with MET followed by increasing β-cell responsivity with GLY or supplementing with insulin might be a more optimal strategy for GDM management than monotherapy.
© 2019 The Authors Clinical Pharmacology & Therapeutics © 2019 American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Conflict of Interest/Disclosure
The authors declare that at the time of the conduct and analysis of the study, they had no competing interests for this work. However, since the completion of the study, D.L.S.’s affiliation has changed to PRAHealthSciences.
Figures


References
-
- Jovanovic L, Pettitt DJ Gestational diabetes mellitus. J.A.M.A 286, 2516–2518 (2001). - PubMed
-
- Paglia MJ, Coustan DR Gestational diabetes: evolving diagnostic criteria. Curr. Opin. Obste.t Gynecol. 23, 72–75 (2011). - PubMed
-
- The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Int J Gynaecol Obstet 78, 69–77 (2002). - PubMed
-
- Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet. Gynecol 131(2):e49–e64 (2018). - PubMed
-
- Committee on Practice, Bulletins-Obstetrics. Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet. Gynecol 122, 406–416 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

