Bacterial steroid-17,20-desmolase is a taxonomically rare enzymatic pathway that converts prednisone to 1,4-androstanediene-3,11,17-trione, a metabolite that causes proliferation of prostate cancer cells
- PMID: 31870912
- PMCID: PMC7333170
- DOI: 10.1016/j.jsbmb.2019.105567
Bacterial steroid-17,20-desmolase is a taxonomically rare enzymatic pathway that converts prednisone to 1,4-androstanediene-3,11,17-trione, a metabolite that causes proliferation of prostate cancer cells
Abstract
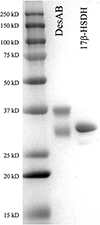
The adrenal gland has traditionally been viewed as a source of "weak androgens"; however, emerging evidence indicates 11-oxy-androgens of adrenal origin are metabolized in peripheral tissues to potent androgens. Also emerging is the role of gut bacteria in the conversion of C21 glucocorticoids to 11-oxygenated C19 androgens. Clostridium scindens ATCC 35,704 is a gut microbe capable of converting cortisol into 11-oxy-androgens by cleaving the side-chain. The desA and desB genes encode steroid-17,20-desmolase. Our prior study indicated that the urinary tract bacterium, Propionimicrobium lymphophilum ACS-093-V-SCH5 encodes desAB and converts cortisol to 11β-hydroxyandrostenedione. We wanted to determine how widespread this function occurs in the human microbiome. Phylogenetic and sequence similarity network analyses indicated that the steroid-17,20-desmolase pathway is taxonomically rare and located in gut and urogenital microbiomes. Two microbes from each of these niches, C. scindens and Propionimicrobium lymphophilum, respectively, were screened for activity against endogenous (cortisol, cortisone, and allotetrahydrocortisol) and exogenous (prednisone, prednisolone, dexamethasone, and 9-fluorocortisol) glucocorticoids. LC/MS analysis showed that both microbes were able to side-chain cleave all glucocorticoids, forming 11-oxy-androgens. Pure recombinant DesAB from C. scindens showed the highest activity against prednisone, a commonly prescribed glucocorticoid. In addition, 0.1 nM 1,4-androstadiene-3,11,17-trione, bacterial side-chain cleavage product of prednisone, showed significant proliferation relative to vehicle in androgen-dependent growth LNCaP prostate cancer cells after 24 h (2.3 fold; P < 0.01) and 72 h (1.6 fold; P < 0.01). Taken together, DesAB-expressing microbes may be an overlooked source of androgens in the body, potentially contributing to various disease states, such as prostate cancer.
Keywords: Microbiome drug metabolism; Prednisone; Prostate cancer; Steroid desmolase.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
An expanded metabolic pathway for androgen production by commensal bacteria.Nat Microbiol. 2025 May;10(5):1084-1098. doi: 10.1038/s41564-025-01979-9. Epub 2025 Apr 21. Nat Microbiol. 2025. PMID: 40259019
-
The desA and desB genes from Clostridium scindens ATCC 35704 encode steroid-17,20-desmolase.J Lipid Res. 2018 Jun;59(6):1005-1014. doi: 10.1194/jlr.M083949. Epub 2018 Mar 23. J Lipid Res. 2018. PMID: 29572237 Free PMC article.
-
Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens.J Lipid Res. 2013 Sep;54(9):2437-49. doi: 10.1194/jlr.M038869. Epub 2013 Jun 15. J Lipid Res. 2013. PMID: 23772041 Free PMC article.
-
Gut feelings about bacterial steroid-17,20-desmolase.Mol Cell Endocrinol. 2021 Apr 5;525:111174. doi: 10.1016/j.mce.2021.111174. Epub 2021 Jan 24. Mol Cell Endocrinol. 2021. PMID: 33503463 Free PMC article. Review.
-
The hunt for a selective 17,20 lyase inhibitor; learning lessons from nature.J Steroid Biochem Mol Biol. 2016 Oct;163:136-46. doi: 10.1016/j.jsbmb.2016.04.021. Epub 2016 May 3. J Steroid Biochem Mol Biol. 2016. PMID: 27154414 Free PMC article. Review.
Cited by
-
Endocrine-Disrupting Chemicals, Gut Microbiota, and Human (In)Fertility-It Is Time to Consider the Triad.Cells. 2022 Oct 22;11(21):3335. doi: 10.3390/cells11213335. Cells. 2022. PMID: 36359730 Free PMC article.
-
Current evidence and clinical relevance of drug-microbiota interactions in inflammatory bowel disease.Front Microbiol. 2023 Feb 23;14:1107976. doi: 10.3389/fmicb.2023.1107976. eCollection 2023. Front Microbiol. 2023. PMID: 36910207 Free PMC article. Review.
-
Microbial metabolism marvels: a comprehensive review of microbial drug transformation capabilities.Gut Microbes. 2024 Jan-Dec;16(1):2387400. doi: 10.1080/19490976.2024.2387400. Epub 2024 Aug 16. Gut Microbes. 2024. PMID: 39150897 Free PMC article. Review.
-
Interplay between inflammatory bowel disease therapeutics and the gut microbiome reveals opportunities for novel treatment approaches.Microbiome Res Rep. 2023 Sep 26;2(4):35. doi: 10.20517/mrr.2023.41. Microbiome Res Rep. 2023. PMID: 37849974 Free PMC article.
-
Another renaissance for bile acid gastrointestinal microbiology.Nat Rev Gastroenterol Hepatol. 2024 May;21(5):348-364. doi: 10.1038/s41575-024-00896-2. Epub 2024 Feb 21. Nat Rev Gastroenterol Hepatol. 2024. PMID: 38383804 Free PMC article. Review.
References
-
- Pretorius E, Arlt W, Storbeck KH. 2017. A new dawn for androgens: Novel lessons from 11-oxygenated C19 steroids. Mol Cell Endocrinol 441 (2017) pp. 76–85. - PubMed
-
- Swart AC, Storbeck K. 11β-hydroxyandrostenedione: Downstream metabolism by 11βHSD, 17βHSD and SRD5A produces novel substrates in familiar pathways. Mol Cell Endocrinol 408 (2015) pp. 114–123. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

