VNRX-5133 (Taniborbactam), a Broad-Spectrum Inhibitor of Serine- and Metallo-β-Lactamases, Restores Activity of Cefepime in Enterobacterales and Pseudomonas aeruginosa
- PMID: 31871094
- PMCID: PMC7038240
- DOI: 10.1128/AAC.01963-19
VNRX-5133 (Taniborbactam), a Broad-Spectrum Inhibitor of Serine- and Metallo-β-Lactamases, Restores Activity of Cefepime in Enterobacterales and Pseudomonas aeruginosa
Abstract
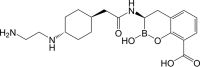
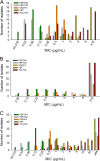
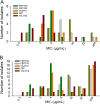
As shifts in the epidemiology of β-lactamase-mediated resistance continue, carbapenem-resistant Enterobacterales (CRE) and carbapenem-resistant Pseudomonas aeruginosa (CRPA) are the most urgent threats. Although approved β-lactam (BL)-β-lactamase inhibitor (BLI) combinations address widespread serine β-lactamases (SBLs), such as CTX-M-15, none provide broad coverage against either clinically important serine-β-lactamases (KPC, OXA-48) or clinically important metallo-β-lactamases (MBLs; e.g., NDM-1). VNRX-5133 (taniborbactam) is a new cyclic boronate BLI that is in clinical development combined with cefepime for the treatment of infections caused by β-lactamase-producing CRE and CRPA. Taniborbactam is the first BLI with direct inhibitory activity against Ambler class A, B, C, and D enzymes. From biochemical and structural analyses, taniborbactam exploits substrate mimicry while employing distinct mechanisms to inhibit both SBLs and MBLs. It is a reversible covalent inhibitor of SBLs with slow dissociation and a prolonged active-site residence time (half-life, 30 to 105 min), while in MBLs, it behaves as a competitive inhibitor, with inhibitor constant (Ki ) values ranging from 0.019 to 0.081 μM. Inhibition is achieved by mimicking the transition state structure and exploiting interactions with highly conserved active-site residues. In microbiological testing, taniborbactam restored cefepime activity in 33/34 engineered Escherichia coli strains overproducing individual enzymes covering Ambler classes A, B, C, and D, providing up to a 1,024-fold shift in the MIC. Addition of taniborbactam restored the antibacterial activity of cefepime against all 102 Enterobacterales clinical isolates tested and 38/41 P. aeruginosa clinical isolates tested with MIC90s of 1 and 4 μg/ml, respectively, representing ≥256- and ≥32-fold improvements, respectively, in antibacterial activity over that of cefepime alone. The data demonstrate the potent, broad-spectrum rescue of cefepime activity by taniborbactam against clinical isolates of CRE and CRPA.
Keywords: antibacterial; biochemistry; microbiology; structural biology; β-lactamases; β-lactams.
Copyright © 2020 Hamrick et al.
Figures



References
-
- CDC. 2019. Antibiotic resistance threats in the United States 2019. CDC, U.S. Department of Health and Human Services, Atlanta, GA: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-re....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

