The Cryptic Plasmid Improves Chlamydia Fitness in Different Regions of the Gastrointestinal Tract
- PMID: 31871102
- PMCID: PMC7035928
- DOI: 10.1128/IAI.00860-19
The Cryptic Plasmid Improves Chlamydia Fitness in Different Regions of the Gastrointestinal Tract
Abstract
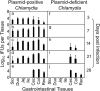
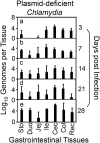
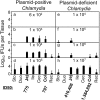
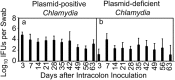
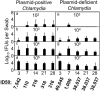
The cryptic plasmid is important for chlamydial colonization in the gastrointestinal tract. We used a combination of intragastric, intrajejunal, and intracolon inoculations to reveal the impact of the plasmid on chlamydial colonization in distinct regions of gastrointestinal tract. Following an intragastric inoculation, the plasmid significantly improved chlamydial colonization. At the tissue level, plasmid-positive Chlamydia produced infectious progenies throughout gastrointestinal tract. However, to our surprise, plasmid-deficient Chlamydia failed to produce infectious progenies in small intestine, although infectious progenies were eventually detected in large intestine, indicating a critical role of the plasmid in chlamydial differentiation into infectious particles in small intestine. The noninfectious status may represent persistent infection, since Chlamydia genomes proliferated in the same tissues. Following an intrajejunal inoculation that bypasses the gastric barrier, plasmid-deficient Chlamydia produced infectious progenies in small intestine but was 530-fold less infectious than plasmid-positive Chlamydia, suggesting that (i) the noninfectious status developed after intragastric inoculation might be induced by a combination of gastric and intestinal effectors and (ii) chlamydial colonization in small intestine was highly dependent on plasmid. Finally, following an intracolon inoculation, the dependence of chlamydial colonization on plasmid increased over time. Thus, we have demonstrated that the plasmid may be able to improve chlamydial fitness in different gut regions via different mechanisms, which has laid a foundation to further reveal the specific mechanisms.
Keywords: Chlamydia; Chlamydia muridarum; GI tract colonization; gastrointestinal tract; persistence; plasmid.
Copyright © 2020 American Society for Microbiology.
Figures
Similar articles
-
Chlamydia overcomes multiple gastrointestinal barriers to achieve long-lasting colonization.Trends Microbiol. 2021 Nov;29(11):1004-1012. doi: 10.1016/j.tim.2021.03.011. Epub 2021 Apr 14. Trends Microbiol. 2021. PMID: 33865675 Free PMC article. Review.
-
The cryptic plasmid is more important for Chlamydia muridarum to colonize the mouse gastrointestinal tract than to infect the genital tract.PLoS One. 2017 May 25;12(5):e0177691. doi: 10.1371/journal.pone.0177691. eCollection 2017. PLoS One. 2017. PMID: 28542376 Free PMC article.
-
The Genital Tract Virulence Factor pGP3 Is Essential for Chlamydia muridarum Colonization in the Gastrointestinal Tract.Infect Immun. 2017 Dec 19;86(1):e00429-17. doi: 10.1128/IAI.00429-17. Print 2018 Jan. Infect Immun. 2017. PMID: 29038127 Free PMC article.
-
Chlamydia Deficient in Plasmid-Encoded pGP3 Is Prevented from Spreading to Large Intestine.Infect Immun. 2020 May 20;88(6):e00120-20. doi: 10.1128/IAI.00120-20. Print 2020 May 20. Infect Immun. 2020. PMID: 32205401 Free PMC article.
-
Chlamydial Plasmid-Dependent Pathogenicity.Trends Microbiol. 2017 Feb;25(2):141-152. doi: 10.1016/j.tim.2016.09.006. Epub 2016 Oct 3. Trends Microbiol. 2017. PMID: 27712952 Free PMC article. Review.
Cited by
-
Chlamydia muridarum mutant CM-pGP3S as a novel attenuated live rectal vaccine protects against genital tract infection.Front Cell Infect Microbiol. 2025 May 13;15:1550455. doi: 10.3389/fcimb.2025.1550455. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40433661 Free PMC article.
-
Infection of human organoids supports an intestinal niche for Chlamydia trachomatis.PLoS Pathog. 2024 Aug 22;20(8):e1012144. doi: 10.1371/journal.ppat.1012144. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39172739 Free PMC article.
-
Chlamydia overcomes multiple gastrointestinal barriers to achieve long-lasting colonization.Trends Microbiol. 2021 Nov;29(11):1004-1012. doi: 10.1016/j.tim.2021.03.011. Epub 2021 Apr 14. Trends Microbiol. 2021. PMID: 33865675 Free PMC article. Review.
-
Gastrointestinal Chlamydia-Induced CD8+ T Cells Promote Chlamydial Pathogenicity in the Female Upper Genital Tract.Infect Immun. 2021 Sep 16;89(10):e0020521. doi: 10.1128/IAI.00205-21. Epub 2021 Jul 6. Infect Immun. 2021. PMID: 34227838 Free PMC article.
-
Robust Heat Shock Response in Chlamydia Lacking a Typical Heat Shock Sigma Factor.Front Microbiol. 2022 Jan 3;12:812448. doi: 10.3389/fmicb.2021.812448. eCollection 2021. Front Microbiol. 2022. PMID: 35046926 Free PMC article.
References
-
- de la Maza LM, Peterson EM. 2002. Vaccines for Chlamydia trachomatis infections. Curr Opin Invest Drugs 3:980–986. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

