High-Throughput Prediction of MHC Class I and II Neoantigens with MHCnuggets
- PMID: 31871119
- PMCID: PMC7056596
- DOI: 10.1158/2326-6066.CIR-19-0464
High-Throughput Prediction of MHC Class I and II Neoantigens with MHCnuggets
Abstract
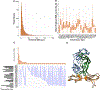
Computational prediction of binding between neoantigen peptides and major histocompatibility complex (MHC) proteins can be used to predict patient response to cancer immunotherapy. Current neoantigen predictors focus on in silico estimation of MHC binding affinity and are limited by low predictive value for actual peptide presentation, inadequate support for rare MHC alleles, and poor scalability to high-throughput data sets. To address these limitations, we developed MHCnuggets, a deep neural network method that predicts peptide-MHC binding. MHCnuggets can predict binding for common or rare alleles of MHC class I or II with a single neural network architecture. Using a long short-term memory network (LSTM), MHCnuggets accepts peptides of variable length and is faster than other methods. When compared with methods that integrate binding affinity and MHC-bound peptide (HLAp) data from mass spectrometry, MHCnuggets yields a 4-fold increase in positive predictive value on independent HLAp data. We applied MHCnuggets to 26 cancer types in The Cancer Genome Atlas, processing 26.3 million allele-peptide comparisons in under 2.3 hours, yielding 101,326 unique predicted immunogenic missense mutations (IMM). Predicted IMM hotspots occurred in 38 genes, including 24 driver genes. Predicted IMM load was significantly associated with increased immune cell infiltration (P < 2 × 10-16), including CD8+ T cells. Only 0.16% of predicted IMMs were observed in more than 2 patients, with 61.7% of these derived from driver mutations. Thus, we describe a method for neoantigen prediction and its performance characteristics and demonstrate its utility in data sets representing multiple human cancers.
©2019 American Association for Cancer Research.
Conflict of interest statement
Disclosure of potential conflicts of interest
The terms of these arrangements are managed by Johns Hopkins University in accordance with its conflict of interest policies.
Potential Conflicts of Interest: The terms of these arrangements are managed by Johns Hopkins University in accordance with its conflict of interest policies.
Figures



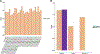

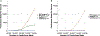
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

