High-Fat Diet-Induced Obesity Causes Sex-Specific Deficits in Adult Hippocampal Neurogenesis in Mice
- PMID: 31871124
- PMCID: PMC6946541
- DOI: 10.1523/ENEURO.0391-19.2019
High-Fat Diet-Induced Obesity Causes Sex-Specific Deficits in Adult Hippocampal Neurogenesis in Mice
Abstract
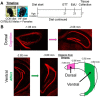
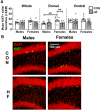
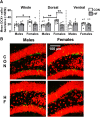
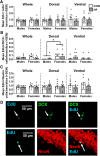
Adult hippocampal neurogenesis (AHN) is suppressed by high-fat (HF) diet and metabolic disease, including obesity and type 2 diabetes. Deficits in AHN may contribute to cognitive decline and increased risk of dementia and mood disorders, which have higher prevalence in women. However, sex differences in the effects of HF diet/metabolic disease on AHN have yet to be thoroughly investigated. Herein, male and female C57BL/6J mice were fed an HF or control (CON) diet from ∼2 to 6 months of age. After 3 months on the diet, mice were injected with 5-ethynyl-2'-deoxyuridine (EdU) then killed 4 weeks later. Cell proliferation, differentiation/maturation, and survival of new neurons in the dentate gyrus were assessed with immunofluorescence for EdU, Ki67, doublecortin (DCX), and NeuN. CON females had more proliferating cells (Ki67+) and neuroblasts/immature neurons (DCX+) compared with CON males; however, HF diet reduced these cells in females to the levels of males. Diet did not affect neurogenesis in males. Further, the numbers of proliferating cells and immature neurons were inversely correlated with both weight gain and glucose intolerance in females only. These effects were robust in the dorsal hippocampus, which supports cognitive processes. Assessment of microglia in the dentate gyrus using immunofluorescence for Iba1 and CD68 uncovered sex-specific effects of diet, which may contribute to observed differences in neurogenesis. These findings demonstrate sex-specific effects of HF diet/metabolic disease on AHN, and highlight the potential for targeting neurogenic deficits to treat cognitive decline and reduce the risk of dementia associated with these conditions, particularly in females.
Keywords: hippocampus; metabolic disorders; microglia; neurogenesis; prediabetes; sex differences.
Copyright © 2020 Robison et al.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
