Biodegradable nanofiber-based piezoelectric transducer
- PMID: 31871178
- PMCID: PMC6955346
- DOI: 10.1073/pnas.1910343117
Biodegradable nanofiber-based piezoelectric transducer
Abstract
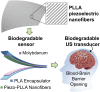
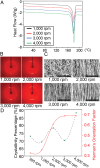
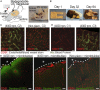
Piezoelectric materials, a type of "smart" material that generates electricity while deforming and vice versa, have been used extensively for many important implantable medical devices such as sensors, transducers, and actuators. However, commonly utilized piezoelectric materials are either toxic or nondegradable. Thus, implanted devices employing these materials raise a significant concern in terms of safety issues and often require an invasive removal surgery, which can damage directly interfaced tissues/organs. Here, we present a strategy for materials processing, device assembly, and electronic integration to 1) create biodegradable and biocompatible piezoelectric PLLA [poly(l-lactic acid)] nanofibers with a highly controllable, efficient, and stable piezoelectric performance, and 2) demonstrate device applications of this nanomaterial, including a highly sensitive biodegradable pressure sensor for monitoring vital physiological pressures and a biodegradable ultrasonic transducer for blood-brain barrier opening that can be used to facilitate the delivery of drugs into the brain. These significant applications, which have not been achieved so far by conventional piezoelectric materials and bulk piezoelectric PLLA, demonstrate the PLLA nanofibers as a powerful material platform that offers a profound impact on various medical fields including drug delivery, tissue engineering, and implanted medical devices.
Keywords: PLLA; biodegradable; piezoelectric; pressure sensors; ultrasound transducer.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Chorsi M. T., et al. , Piezoelectric biomaterials for sensors and actuators. Adv. Mater. 31, e1802084 (2019). - PubMed
-
- Nguyen T. D., et al. , Piezoelectric nanoribbons for monitoring cellular deformations. Nat. Nanotechnol. 7, 587–593 (2012). - PubMed
-
- Ikada Y., Shikinami Y., Hara Y., Tagawa M., Fukada E., Enhancement of bone formation by drawn poly(l-lactide). J. Biomed. Mater. Res. 30, 553–558 (1996). - PubMed
-
- Tajitsu Y., Kanesaki M., Tsukiji M., Imoto K., Date M., Fukada E., Novel tweezers for biological cells using piezoelectric polylactic acid fibers. Ferroelectrics 320, 133–139 (2005).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

