The olfactory basis of orchid pollination by mosquitoes
- PMID: 31871198
- PMCID: PMC6955360
- DOI: 10.1073/pnas.1910589117
The olfactory basis of orchid pollination by mosquitoes
Abstract
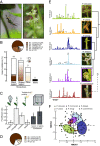
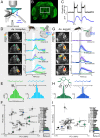
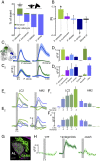
Mosquitoes are important vectors of disease and require sources of carbohydrates for reproduction and survival. Unlike host-related behaviors of mosquitoes, comparatively less is understood about the mechanisms involved in nectar-feeding decisions, or how this sensory information is processed in the mosquito brain. Here we show that Aedes spp. mosquitoes, including Aedes aegypti, are effective pollinators of the Platanthera obtusata orchid, and demonstrate this mutualism is mediated by the orchid's scent and the balance of excitation and inhibition in the mosquito's antennal lobe (AL). The P. obtusata orchid emits an attractive, nonanal-rich scent, whereas related Platanthera species-not visited by mosquitoes-emit scents dominated by lilac aldehyde. Calcium imaging experiments in the mosquito AL revealed that nonanal and lilac aldehyde each respectively activate the LC2 and AM2 glomerulus, and remarkably, the AM2 glomerulus is also sensitive to N,N-diethyl-meta-toluamide (DEET), a mosquito repellent. Lateral inhibition between these 2 glomeruli reflects the level of attraction to the orchid scents. Whereas the enriched nonanal scent of P. obtusata activates the LC2 and suppresses AM2, the high level of lilac aldehyde in the other orchid scents inverts this pattern of glomerular activity, and behavioral attraction is lost. These results demonstrate the ecological importance of mosquitoes beyond operating as disease vectors and open the door toward understanding the neural basis of mosquito nectar-seeking behaviors.
Keywords: Aedes aegypti; Platanthera; mosquitoes; nectar; olfaction.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: A provisional patent on the odor that mimics the orchid scent was recently filed (62/808,710).
Figures

Similar articles
-
Insect repellents mediate species-specific olfactory behaviours in mosquitoes.Malar J. 2020 Mar 30;19(1):127. doi: 10.1186/s12936-020-03206-8. Malar J. 2020. PMID: 32228701 Free PMC article.
-
The ovipositor cue indole inhibits animal host attraction in Aedes aegypti (Diptera: Culicidae) mosquitoes.Parasit Vectors. 2022 Nov 12;15(1):422. doi: 10.1186/s13071-022-05545-8. Parasit Vectors. 2022. PMID: 36369215 Free PMC article.
-
orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET.Nature. 2013 Jun 27;498(7455):487-91. doi: 10.1038/nature12206. Epub 2013 May 29. Nature. 2013. PMID: 23719379 Free PMC article.
-
Olfactory coding in the mosquito antennal lobe: labeled lines or combinatorial code?Curr Opin Insect Sci. 2025 Apr;68:101299. doi: 10.1016/j.cois.2024.101299. Epub 2024 Nov 15. Curr Opin Insect Sci. 2025. PMID: 39550060 Review.
-
Orchid pollination by sexual deception: pollinator perspectives.Biol Rev Camb Philos Soc. 2011 Feb;86(1):33-75. doi: 10.1111/j.1469-185X.2010.00134.x. Biol Rev Camb Philos Soc. 2011. PMID: 20377574 Review.
Cited by
-
Internal and external drivers interact to create highly dynamic mosquito blood-feeding behaviour.Proc Biol Sci. 2024 Aug;291(2029):20241105. doi: 10.1098/rspb.2024.1105. Epub 2024 Aug 28. Proc Biol Sci. 2024. PMID: 39196275 Free PMC article. Review.
-
Synthesis and Olfactory Properties of Seco-Analogues of Lilac Aldehydes.Molecules. 2021 Nov 23;26(23):7086. doi: 10.3390/molecules26237086. Molecules. 2021. PMID: 34885667 Free PMC article.
-
Avoidance of the Plant Hormone Cis-Jasmone by Aedes aegypti Depends On Mosquito Age in Both Plant and Human Odor Backgrounds.J Chem Ecol. 2021 Sep;47(8-9):810-818. doi: 10.1007/s10886-021-01299-2. Epub 2021 Aug 31. J Chem Ecol. 2021. PMID: 34463894 Free PMC article.
-
We know very little about pollination in the Platanthera Rich (Orchidaceae: Orchidoideae).Ecol Evol. 2024 Apr 10;14(4):e11223. doi: 10.1002/ece3.11223. eCollection 2024 Apr. Ecol Evol. 2024. PMID: 38606342 Free PMC article. Review.
-
Afrotropical sand fly-host plant relationships in a leishmaniasis endemic area, Kenya.PLoS Negl Trop Dis. 2021 Feb 8;15(2):e0009041. doi: 10.1371/journal.pntd.0009041. eCollection 2021 Feb. PLoS Negl Trop Dis. 2021. PMID: 33556068 Free PMC article.
References
-
- World Health Organization , “A global brief on vector-borne diseases” (No. WHO/DCO/WHD/2014.1, WHO Press, Geneva, Switzerland, 2014).
-
- Cardé R. T., Gibson G., Host finding by female mosquitoes: mechanisms of orientation to host odours and other cues. Olfaction vector host Interact. 2010, 115–142 (2010).
-
- Takken W., Verhulst N. O., Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 58, 433–453 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

