Alzheimer's-like pathology in aging rhesus macaques: Unique opportunity to study the etiology and treatment of Alzheimer's disease
- PMID: 31871209
- PMCID: PMC6936707
- DOI: 10.1073/pnas.1903671116
Alzheimer's-like pathology in aging rhesus macaques: Unique opportunity to study the etiology and treatment of Alzheimer's disease
Abstract
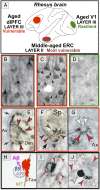
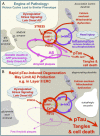
Although mouse models of Alzheimer's disease (AD) have provided tremendous breakthroughs, the etiology of later onset AD remains unknown. In particular, tau pathology in the association cortex is poorly replicated in mouse models. Aging rhesus monkeys naturally develop cognitive deficits, amyloid plaques, and the same qualitative pattern and sequence of tau pathology as humans, with tangles in the oldest animals. Thus, aging rhesus monkeys can play a key role in AD research. For example, aging monkeys can help reveal how synapses in the prefrontal association cortex are uniquely regulated compared to the primary sensory cortex in ways that render them vulnerable to calcium dysregulation and tau phosphorylation, resulting in the selective localization of tau pathology observed in AD. The ability to assay early tau phosphorylation states and perform high-quality immunoelectron microscopy in monkeys is a great advantage, as one can capture early-stage degeneration as it naturally occurs in situ. Our immunoelectron microscopy studies show that phosphorylated tau can induce an "endosomal traffic jam" that drives amyloid precursor protein cleavage to amyloid-β in endosomes. As amyloid-β increases tau phosphorylation, this creates a vicious cycle where varied precipitating factors all lead to a similar phenotype. These data may help explain why circuits with aggressive tau pathology (e.g., entorhinal cortex) may degenerate prior to producing significant amyloid pathology. Aging monkeys therefore can play an important role in identifying and testing potential therapeutics to protect the association cortex, including preventive therapies that are challenging to test in humans.
Keywords: PKA; entorhinal cortex; prefrontal cortex; ryanodine.
Conflict of interest statement
Competing interest statement: A.F.T.A. and Yale University receive royalties from the United States sales of Intuniv by Shire/Takeda Pharmaceuticals. They do not receive royalties from generic or international sales.
Figures



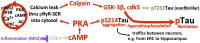

References
-
- Hersi M., et al. , Risk factors associated with the onset and progression of Alzheimer’s disease: A systematic review of the evidence. Neurotoxicology 61, 143–187 (2017). - PubMed
-
- Braak H., Zetterberg H., Del Tredici K., Blennow K., Intraneuronal tau aggregation precedes diffuse plaque deposition, but amyloid-β changes occur before increases of tau in cerebrospinal fluid. Acta Neuropathol. 126, 631–641 (2013). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

