Pharmacokinetic Parameters of Quinine in Healthy Subjects and in Patients with Uncomplicated Malaria in Nigeria: Analysis of Data using a Population Approach
- PMID: 31871506
- PMCID: PMC6911901
- DOI: 10.1016/j.curtheres.2019.100567
Pharmacokinetic Parameters of Quinine in Healthy Subjects and in Patients with Uncomplicated Malaria in Nigeria: Analysis of Data using a Population Approach
Abstract
Background: The varied disposition of the antimalarial quinine partly explains its poor tolerance and toxicity in humans.
Objective: Using a population approach, the disposition of quinine in healthy subjects and patients with acute uncomplicated symptomatic malaria from Nigeria was re-examined with a view to providing population-specific attributes.
Methods: Concentration versus time profiles of quinine over 48 hours in healthy individuals, and over 7 days in malaria-infected patients, were stratified to reflect: concentration versus time data during the first 48 hours of quinine administration for healthy subjects and infected patients, concentration versus time data after 48 hours in infected patients, and all concentration versus time data available for healthy subjects and infected patients. Pharmacokinetic parameters were then estimated with a stochastic approximation expectation maximization algorithm.
Results: All datasets were fitted by a 1-compartment model with covariate contributions from body weight and infection status. The absorption rate constant, and volume of distribution and clearance were 1.72 h-1, 86.8 to 157.4 L, and 6.6 to 9.6 L/h, respectively. Infected patients experienced a 38% decrease in volume of distribution and a 31% decrease in clearance in the first 48 hours relative to healthy individuals. The contraction in volume of distribution and clearance diminished significantly after 48 hours of chronic quinine dosing in infected patients.
Conclusions: The study findings suggest that clinical interventions aimed at enhancing the safety and tolerance of quinine might be achieved by a rational decrease in dose size and/or dosing interval, post-48 hours of chronic quinine administration, in malaria-infected patients.
Keywords: Nigerians; malaria; population pharmacokinetics; quinine; tolerance; toxicity.
© 2019 The Author(s).
Conflict of interest statement
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Figures
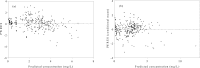
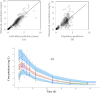
References
-
- World Health Organisation . Third Edition. 2015. Guidelines for the treatment of malaria.
-
- Salako L.A., Sowunmi A. Disposition of quinine in plasma, red blood cells and saliva after oral and intravenous administration to healthy adult Africans. Eur J Clin Pharmacol. 1992;42(2):171–174. - PubMed
-
- Fuder H., Herzog R., Vaupel W., Wetzelsberger N., Lucker P.W. Study on the absolute bioavailability of quinine and theophylline from tablets after single dose oral administration as compared to intravenous infusion in healthy male non-smoking volunteers. Methods Find Exp Clin Pharmacol. 1994;16(9):651–660. - PubMed
LinkOut - more resources
Full Text Sources

