A joint molecular networking study of a Smenospongia sponge and a cyanobacterial bloom revealed new antiproliferative chlorinated polyketides
- PMID: 31871685
- PMCID: PMC6927677
- DOI: 10.1039/C9QO00074G
A joint molecular networking study of a Smenospongia sponge and a cyanobacterial bloom revealed new antiproliferative chlorinated polyketides
Abstract
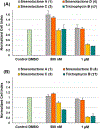
The bloom-forming cyanobacteria Trichodesmium sp. have been recently shown to produce some of the chlorinated peptides/polyketides previously isolated from the marine sponge Smenospongia aurea. A comparative analysis of extracts from S. aurea and Trichodesmium sp. was performed using tandem mass spectrometry-based molecular networking. The analysis, specifically targeted to chlorinated metabolites, showed that many of them are common to the two organisms, but also that some general differences exist between the two metabolomes. Following this analysis, six new chlorinated metabolites were isolated and their structures elucidated: four polyketides, smenolactones A-D (1-4) from S. aurea, and two new conulothiazole analogues, isoconulothiazole B (5) and conulothiazole C (6) from Trichodesmium sp. The absolute configuration of smenolactone C (3) was determined by taking advantage of the conformational rigidity of open 1,3-disubstituted alkyl chains. The antiproliferative activity of smenolactones was evaluated on three tumor cell lines, and they were active at low-micromolar or sub-micromolar concentrations.
Conflict of interest statement
Conflicts of interest There are no conflicts to declare
Figures



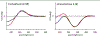



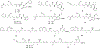
References
-
- Esposito G, Bourguet-Kondracki M-L, Mai LH, Longeon A, Teta R, Meijer L, Van Soest R, Mangoni A and Costantino V, J. Nat. Prod, 2016, 79, 2953–2960. - PubMed
-
- Costantino V, Fattorusso E, Imperatore C and Mangoni A, European J. Org. Chem, 2003, 1433–1437.
-
- Costantino V, Fattorusso E, Imperatore C, Mangoni A and Teta R, European J. Org. Chem, 2009, 2112–2119.
Grants and funding
LinkOut - more resources
Full Text Sources
