Extracellular Vesicles in Cancer Immune Microenvironment and Cancer Immunotherapy
- PMID: 31871860
- PMCID: PMC6918121
- DOI: 10.1002/advs.201901779
Extracellular Vesicles in Cancer Immune Microenvironment and Cancer Immunotherapy
Abstract
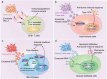
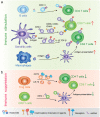
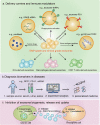
Extracellular vesicles (EVs) are secreted by almost all cells. They contain proteins, lipids, and nucleic acids which are delivered from the parent cells to the recipient cells. Thereby, they function as mediators of intercellular communication and molecular transfer. Recent evidences suggest that exosomes, a small subset of EVs, are involved in numerous physiological and pathological processes and play essential roles in remodeling the tumor immune microenvironment even before the occurrence and metastasis of cancer. Exosomes derived from tumor cells and host cells mediate their mutual regulation locally or remotely, thereby determining the responsiveness of cancer therapies. As such, tumor-derived circulating exosomes are considered as noninvasive biomarkers for early detection and diagnosis of tumor. Exosome-based therapies are also emerging as cutting-edge and promising strategies that could be applied to suppress tumor progression or enhance anti-tumor immunity. Herein, the current understanding of exosomes and their key roles in modulating immune responses, as well as their potential therapeutic applications are outlined. The limitations of current studies are also presented and directions for future research are described.
Keywords: anti‐tumor immunity; cancer immunotherapy; exosomes; extracellular vesicles; tumor microenvironment.
© 2019 The Authors. Published by WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Trams E. G., Lauter C. J., Salem N. Jr., Heine U., Biochim. Biophys. Acta, Biomembr. 1981, 645, 63. - PubMed
-
- Simons M., Raposo G., Curr. Opin. Cell Biol. 2009, 21, 575. - PubMed
-
- Thery C., Ostrowski M., Segura E., Nat. Rev. Immunol. 2009, 9, 581. - PubMed
-
- Zhang H., Freitas D., Kim H. S., Fabijanic K., Li Z., Chen H., Mark M. T., Molina H., Martin A. B., Bojmar L., Fang J., Rampersaud S., Hoshino A., Matei I., Kenific C. M., Nakajima M., Mutvei A. P., Sansone P., Buehring W., Wang H., Jimenez J. P., Cohen‐Gould L., Paknejad N., Brendel M., Manova‐Todorova K., Magalhaes A., Ferreira J. A., Osorio H., Silva A. M., Massey A., Cubillos‐Ruiz J. R., Galletti G., Giannakakou P., Cuervo A. M., Blenis J., Schwartz R., Brady M. S., Peinado H., Bromberg J., Matsui H., Reis C. A., Lyden D., Nat. Cell Biol. 2018, 20, 332. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
