Minimally invasive photoacoustic imaging: Current status and future perspectives
- PMID: 31871889
- PMCID: PMC6909166
- DOI: 10.1016/j.pacs.2019.100146
Minimally invasive photoacoustic imaging: Current status and future perspectives
Abstract
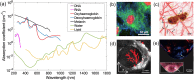
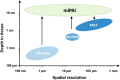
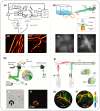
Photoacoustic imaging (PAI) is an emerging biomedical imaging modality that is based on optical absorption contrast, capable of revealing distinct spectroscopic signatures of tissue at high spatial resolution and large imaging depths. However, clinical applications of conventional non-invasive PAI systems have been restricted to examinations of tissues at depths less than a few cm due to strong light attenuation. Minimally invasive photoacoustic imaging (miPAI) has greatly extended the landscape of PAI by delivering excitation light within tissue through miniature fibre-optic probes. In the past decade, various miPAI systems have been developed with demonstrated applicability in several clinical fields. In this article, we present an overview of the current status of miPAI and our thoughts on future perspectives.
Keywords: Interventional photoacoustic imaging; Minimally invasive procedures; Multi-modal imaging; Photoacoustic computed tomography; Photoacoustic endoscopy; Photoacoustic imaging; Photoacoustic microscopy.
© 2019 The Authors.
Conflict of interest statement
The authors declare that there are no conflicts of interest. A.E.D. is a Director and Shareholder of Echopoint Medical, London, UK, and T.V. holds shares from Mauna Kea Technologies, Paris, France, which, however, did not support this work.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

