Missing tumor measurement (TM) data in the search for alternative TM-based endpoints in cancer clinical trials
- PMID: 31872158
- PMCID: PMC6909186
- DOI: 10.1016/j.conctc.2019.100492
Missing tumor measurement (TM) data in the search for alternative TM-based endpoints in cancer clinical trials
Abstract
Purpose: Missing data commonly occur in cancer clinical trials (CCT) and may hinder the search for alternative trial endpoints. We consider reasons for missing tumor measurement (TM) data in CCT and how missing TM data are typically handled. We explore the potential impact of missing TM data on predictive ability of a set of TM-based endpoints.
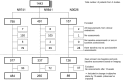
Methods: Literature review identifies reasons for and approaches to handling missing TM data. Data from 3 actual clinical trials were used for illustration. A sensitivity analysis of the potential impact of missing TM data was performed by comparing overall survival (OS) predictive ability of alternative endpoints using observed and imputed data.
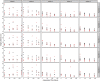
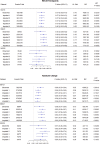
Results: Reasons for missing TM data in CCT are presented, based on the literature review and the three trials. Although missing TM data impacted individual objective status (e.g. 12-week status changed for 53% of patients in one imputation set), it surprisingly only minimally impacted endpoint predictive ability (e.g. median c-indices of 500 imputed datasets ranged from 0.566 to 0.570 for N9741, 0.592-0.616 for N9841, and 0.542-0.624 for N0026).
Conclusion: By understanding the reasons for missingness, we can better anticipate them and minimize their occurrence. Our preliminary analysis suggests missing TM data may not impact endpoint predictive ability, but could impact objective response status classification; however these findings require further validation. With response status accepted as an important phase II endpoint in the development of new cancer therapies (including immunotherapy), we urge that in CCT complete TM data collection and adherence to protocol-defined disease evaluation as closely as possible be a priority.
Keywords: Cancer trials; Missing data; Phase II; Tumor measurement-based endpoints.
© 2019 The Authors. Published by Elsevier Inc.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures




References
-
- Kola I., Landis J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004;3:711–715. - PubMed
-
- Therasse P., Arbuck S.G., Eisenhauer E.A. New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer Inst. 2000;92:205–216. - PubMed
-
- Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45(2):228–247. - PubMed
-
- Karrison T.G., Maitland M.L., Stadler W.M. Design of phase II cancer trials using a continuous endpoint of change in tumor size: application to a study of Sorafenib and Erlotinib in non–small-cell lung cancer. J. Natl. Cancer Inst. 2007;99(19):1455–1461. - PubMed
-
- Jaki T., Andre V., Su T.L. Designing exploratory cancer trials using change in tumour size as primary endpoint. Stat. Med. 2013;32(15):2544–2554. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

