Enhanced Molecular Tension Sensor Based on Bioluminescence Resonance Energy Transfer (BRET)
- PMID: 31872754
- PMCID: PMC7550199
- DOI: 10.1021/acssensors.9b00796
Enhanced Molecular Tension Sensor Based on Bioluminescence Resonance Energy Transfer (BRET)
Abstract
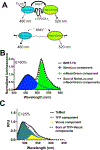
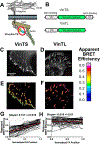
Molecular tension sensors measure piconewton forces experienced by individual proteins in the context of the cellular microenvironment. Current genetically encoded tension sensors use FRET to report on extension of a deformable peptide encoded in a cellular protein of interest. Here, we present the development and characterization of a new type of molecular tension sensor based on bioluminescence resonance energy transfer (BRET), which exhibits more desirable spectral properties and an enhanced dynamic range compared to other molecular tension sensors. Moreover, it avoids many disadvantages of FRET measurements in cells, including autofluorescence, photobleaching, and corrections of direct acceptor excitation. We benchmark the sensor by inserting it into the canonical mechanosensing focal adhesion protein vinculin, observing highly resolved gradients of tensional changes across focal adhesions. We anticipate that the BRET tension sensor will expand the toolkit available to study mechanotransduction at a molecular level and allow potential extension to an in vivo context.
Keywords: BRET; NanoLuc; TSMod; bioluminescence; molecular tension sensor.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

