Efficacy and safety of Chinese patent medicine (Jinlong capsule) in the treatment of advanced hepatocellular carcinoma: a meta-analysis
- PMID: 31872855
- PMCID: PMC6970085
- DOI: 10.1042/BSR20194019
Efficacy and safety of Chinese patent medicine (Jinlong capsule) in the treatment of advanced hepatocellular carcinoma: a meta-analysis
Abstract
Jinlong capsule (JLC), a type of herbal medicine, is considered to be a promising adjuvant therapy for hepatocellular carcinoma (HC). Although an analysis of the published literature has been performed, the exact effects and safety of JLC are yet to be systematically investigated. Therefore, a wide-ranging systematic search of electronic databases to draw conclusions was performed. Data from 29 trials, including 2488 patients with advanced HC, were analyzed. The results indicated that, compared with conventional treatment alone, the combination of conventional treatment and JLC markedly improved overall patient response (odds ratio (OR) 2.06 [95% confidence interval (CI) 1.71-2.49]; P<0.00001), disease control rate (DCR) (OR 2.17 [95% CI 1.74-2.71]; P<0.00001) and quality of life (QoL) (OR 2.71 [95% CI 2.05-3.58]; P<0.00001), and significantly prolonged 6- (P=0.01), 12- (P<0.00001), 24- (P=0.001) and 36-month (P<0.0001) overall survival (OS) rates. The immune function of patients was also significantly enhanced after combined conventional therapy and JLC treatment, indicated by clearly increased percentages of CD3+ (P<0.0001), CD4+ (P<0.00001) and natural killer (NK) cells (P=0.0003), and CD4+/CD8+ ratio (P<0.00001). The incidence of leukopenia (P<0.00001), hepatotoxicity (P=0.005), and myelosuppression (P=0.0007) was lower in HC patients injected with JLC, whereas other adverse events did not differ significantly between the two groups (P>0.05). In summary, results of this meta-analysis suggest that the combination of conventional treatment and JLC is more effective for the treatment of HC than conventional treatment alone.
Keywords: Jinlong Capsule; conventional treatment; hepatocellular carcinoma; meta-analysis; traditional Chinese medicine.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
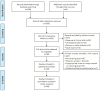




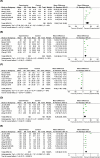
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

